Checkpoint kinase 1 in DNA damage response and cell cycle regulation

DOI 10.1007/s00018-013-1307-3
Cellular and Molecular Life Sciences
Cell. Mol. Life Sci.
Checkpoint kinase 1 in DNA damage response and cell cycle regulation
Mallikarjun Patil · Navjotsingh Pabla · Zheng Dong
Received: 6 November 2012 / Revised: 28 January 2013 / Accepted: 18 February 2013 ? Springer Basel 2013
ATR A TM and Rad3 related Cdc25 C ell division cycle 25CDK C yclin-dependent kinases
Introduction
Checkpoint kinase 1 (Chk1) was initially identified in fis-sion yeast as a serine/threonine protein kinase that is essen-tial for DNA damage-induced cell cycle arrest [1]. Chk1 homologs were subsequently identified in other species such as Drosophila , Xenopus , mouse, and human [2–4]. Functional studies in these model systems showed that Chk1 phosphorylates the key regulators of cyclin-dependent kinase 1 (CDK1) during DNA damage, resulting in CDK1 inactivation and blockade of G2/M transition. More recent work has established important roles of Chk1 not only in DNA damage response (DDR) but also in unperturbed cell cycle. In the normal cell cycle, Chk1 mediates the check-points in S and M phases as well as G2/M transition. In this review, we discuss Chk1 regulation, its role in DDR and unperturbed cell cycle, and the possibility of targeting Chk1 for cancer therapy.
Cell cycle and DNA damage response
The cell cycle consists of a series of highly ordered, sequen-tial phases that lead to cell division [5]. A notable feature of cell cycle progression is that cells do not enter the next phase until the previous phase is completed. This feature, known as cell cycle checkpoints, provides an important sur-veillance mechanism for faithful replication and division of the cells [5] (Fig. 1). Progression from one phase of cell cycle to the next is governed by Cdks, their cyclin partners,
Abstract Originally identified as a mediator of DNA damage response (DDR), checkpoint kinase 1 (Chk1) has a broader role in checkpoint activation in DDR and normal cell cycle regulation. Chk1 activation involves phosphoryla-tion at conserved sites. However, recent work has identi-fied a splice variant of Chk1, which may regulate Chk1 in both DDR and normal cell cycle via molecular interaction. Upon activation, Chk1 phosphorylates a variety of substrate proteins, resulting in the activation of DNA damage check-points, cell cycle arrest, DNA repair, and/or cell death. Chk1 and its related signaling may be an effective therapeutic target in diseases such as cancer.
Keywords Chk1 · Cell cycle · DNA damage · Checkpoint
Abbreviations Chk1 C heckpoint kinase 1Chk2 C heckpoint kinase 2Chk1 S -checkpoint kinase 1-short DDR D NA damage response ATM A taxia telangiectasia mutated
M. Patil · N. Pabla · Z. Dong
Department of Cellular Biology and Anatomy, Georgia Regents University and Charlie Norwood v A Medical Center, 1459 Laney walker Blvd., Augusta, GA 30912, USA
Z. Dong (*)
Department of Nephrology, The Second Xiangya Hospital, Central South University, Changsha 410022, Hunan, China e-mail: zdong@https://www.sodocs.net/doc/0416957312.html,
Present Address: N. Pabla
Department of Pharmaceutical Sciences, St Jude Children’s Research Hospital, Memphis, Tennessee, USA
M. Patil et al.
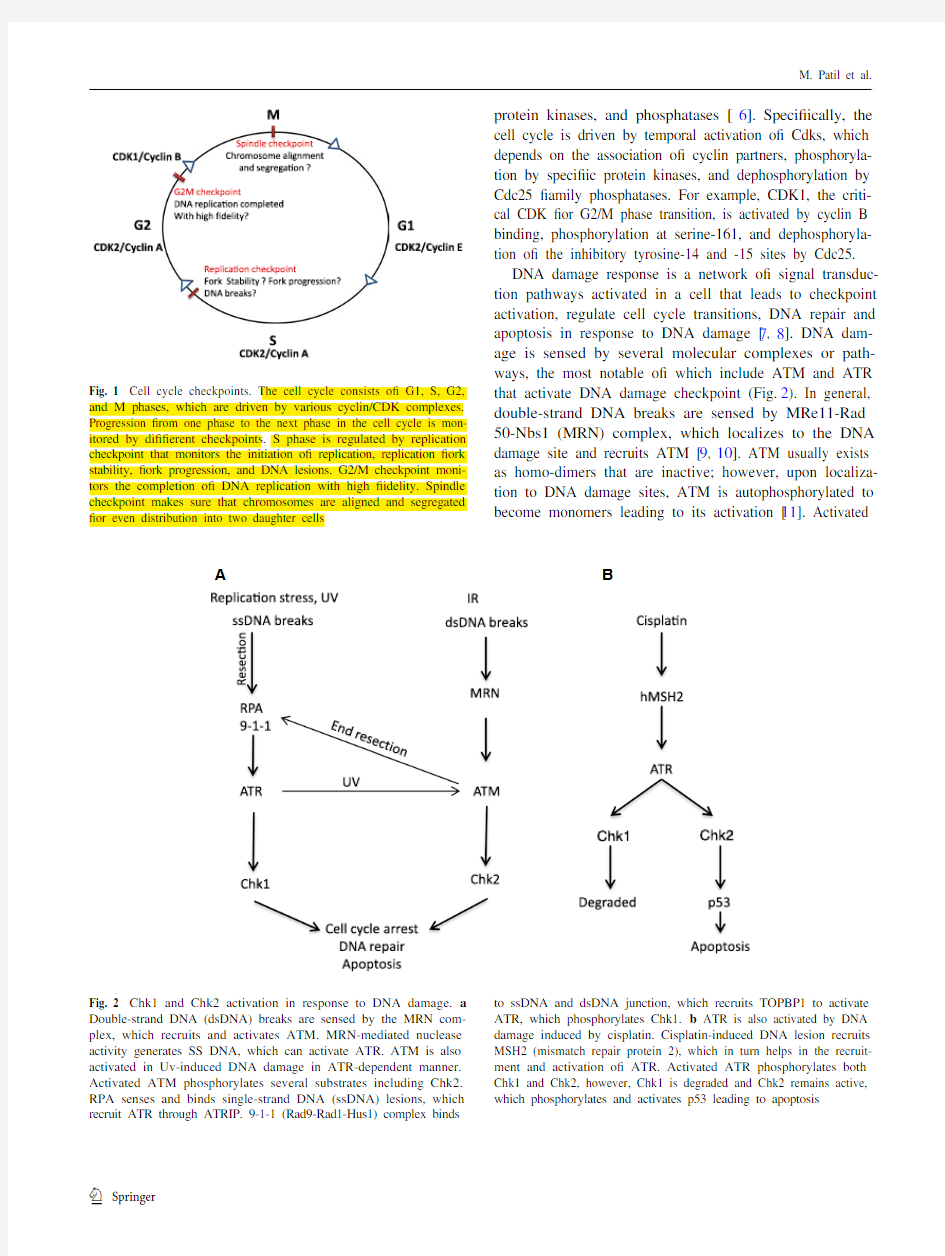
protein kinases, and phosphatases [6]. Specifically, the cell cycle is driven by temporal activation of Cdks, which depends on the association of cyclin partners, phosphoryla-tion by specific protein kinases, and dephosphorylation by Cdc25 family phosphatases. For example, CDK1, the criti-cal CDK for G2/M phase transition, is activated by cyclin B binding, phosphorylation at serine-161, and dephosphoryla-tion of the inhibitory tyrosine-14 and -15 sites by Cdc25.DNA damage response is a network of signal transduc-tion pathways activated in a cell that leads to checkpoint activation, regulate cell cycle transitions, DNA repair and apoptosis in response to DNA damage [7, 8]. DNA dam-age is sensed by several molecular complexes or path-ways, the most notable of which include ATM and ATR that activate DNA damage checkpoint (Fig. 2). In general, double-strand DNA breaks are sensed by MRe11-Rad 50-Nbs1 (MRN) complex, which localizes to the DNA damage site and recruits ATM [9, 10]. ATM usually exists as homo-dimers that are inactive; however, upon localiza-tion to DNA damage sites, ATM is autophosphorylated to become monomers leading to its activation [11
]. Activated
Fig. 1 Cell cycle checkpoints. The cell cycle consists of G1, S, G2, and M phases, which are driven by various cyclin/CDK complexes. Progression from one phase to the next phase in the cell cycle is mon-itored by different checkpoints. S phase is regulated by replication checkpoint that monitors the initiation of replication, replication fork stability, fork progression, and DNA lesions. G2/M checkpoint moni-tors the completion of DNA replication with high fidelity. Spindle checkpoint makes sure that chromosomes are aligned and segregated for even distribution into two daughter cells
A B
Fig. 2 Chk1 and Chk2 activation in response to DNA damage. a Double-strand DNA (dsDNA) breaks are sensed by the MRN com-plex, which recruits and activates ATM. MRN-mediated nuclease activity generates SS DNA, which can activate ATR. ATM is also activated in Uv-induced DNA damage in ATR-dependent manner. Activated ATM phosphorylates several substrates including Chk2. RPA senses and binds single-strand DNA (ssDNA) lesions, which recruit ATR through ATRIP. 9-1-1 (Rad9-Rad1-Hus1) complex binds
to ssDNA and dsDNA junction, which recruits TOPBP1 to activate ATR, which phosphorylates Chk1. b ATR is also activated by DNA damage induced by cisplatin. Cisplatin-induced DNA lesion recruits MSH2 (mismatch repair protein 2), which in turn helps in the recruit-ment and activation of ATR. Activated ATR phosphorylates both Chk1 and Chk2, however, Chk1 is degraded and Chk2 remains active, which phosphorylates and activates p53 leading to apoptosis
Chk1 in DNA damage and cell cycle
ATM phosphorylates downstream targets including Chk2, H2AX, and MDC1 etc. [12]. Different from ATM, ATR is mainly activated by single-stand DNA generated as a result of replication stress, Uv induced DNA damage, nuclease activity consequent to double-strand breaks, enzymatic and helicase remodelling following ICL [13]. The single-strand DNA is recognized and bound by rep-lication protein A (RPA), resulting in the recruitment of ATR through its interacting partner protein called ATRIP [7, 14, 15]. Independently, RAD9-RAD1-HUS1 (9-1-1) complex binds to the ssDNA and dsDNA junction through Rad17 [16]. The 9-1-1 complex in turn helps to recruit topoisomerase binding protein 1 (TOPBP1), an activator of ATR, resulting in ATR activation [17, 18]. In addition to this canonical pathway, recent work has revealed a novel pathway of ATR activation involving mismatch repair pro-teins [19] (Fig. 2). Upon activation, ATR can phosphoryl-ate downstream targets including Chk1. ATR-mediated Chk1 phosphorylation also requires another intermediate protein called claspin, which binds and stabilizes activated Chk1. As a result, Chk1 phosphorylation was abolished in the absence of claspin in Xenopus egg extracts [20]. Although ATM and ATR are generally known to respond to double- and single-strand DNA damage respectively, their signaling pathways are not mutually exclusive. For example, double-strand breaks activate ATM, which along with MRe11 nuclease activity, generates single-strand DNA leading to consequent ATR activation [7, 21]. ATR activated in response to Uv-induced DNA damage activate ATM by phosphorylating ATM at its autophosphorylation site S1981 [22]. In addition, the downstream signaling molecules are not exclusively responsive to ATM or ATR. For example, in cisplatin-induced DNA cross-linking and replication stress, both Chk1 and Chk2 are activated in an ATR-dependent manner. while Chk1 is degraded after ini-tial activation, Chk2 phosphorylates p53 leading to its sta-bilization and activation to induce apoptosis [23]. Intertwined relationship between the cell cycle
and DDR
DNA damage response and the cell cycle are two inter-twined cellular processes. On the one hand, as discussed above, DDR leads to cell cycle arrest by activating check-point kinases. On the other hand, an emerging idea is that even the unperturbed cell cycle has a constitutive surveil-lance mechanism that is related to DDR [24, 25]. This is particularly relevant to DNA replication in S-phase, when single-strand DNA (ssDNA) and DNA breaks may be induced in several ways. During replication, template DNA is unwound at the replication fork by DNA helicases result-ing in ssDNA. ssDNA is highly susceptible to damage such as those induced by free radicals. Breaks in ssDNA at the replication fork can be converted to a double-strand break following replication. As a result, both single-strand and double-strand DNA breaks-associated DDR may be activated to slow down the progression of the cell cycle to repair the damage to ensure a faithful DNA replication and genome integrity. In this process, ATR and Chk1 play a critical role in sensing the initial ssDNA breaks to acti-vate DDR. Consistently, deficiency in either ATR or Chk1 leads to embryonic lethality in mice and embryonic stem cells of these models show cell cycle abnormalities, accu-mulate double-stand breaks and fragile site breaks [26–29]. The intertwined relationship between DDR and cell cycle provides an explanation as to why DDR proteins, such as ATR and Chk1, play such critical roles in the unperturbed cell cycle.
Chk1 in cell cycle regulation and tissue physiology Checkpoint kinase 1 was originally identified as a gene that can rescue a CDK1/cdc2 mutant in fission yeast [1]. Constitutive overexpression of Chk1 in fission yeast leads to mitotic delay, whereas loss of Chk1 has no effect on cell cycle progression. However, in the absence of Chk1, the yeast cells become more sensitive to Uv-induced DNA damage and fail to arrest in G2-phase following DNA dam-age [1]. In Drosophila, Grapes (Chk1 homolog in Drosoph-ila) regulates syncytial cell division fidelity, mitotic entry, and cyclin A degradation and as a result, it is indispensable for embryogenesis [3, 30]. In addition, Grapes may delay the accumulation of cyclin B1 in the nucleus to prevent nuclear CDK1 activation and premature entry into mitosis in Drosophila embryos [31, 32], supporting a role of Chk1 in cell cycle in early stages of embryogenesis in Drosophila. In Xenopus eggs, addition of exogenous, active Chk1 delays mitosis whereas immunodepletion of Chk1 results in an early entry into mitosis [2]. Unlike yeast, mammalian cells do not show noticeable phenotypes following Chk1 over-expression; however Chk1 knockdown and inhibition leads to reduced proliferation and cell death [33]. In mice, Chk1 knockout leads to embryonic death in utero at e6.5 stage [28, 29]. Chk1 knockout blastocysts as well as embry-onic stem cells also fail to proliferate and fail to activate the G2/M checkpoint in response to DNA damage and die shortly in culture [28, 29]. embryonic death of Chk1-null mice is independent of p53 since p53-deficiency fail to res-cue the phenotype [28]. Although viable, Chk1-deficient chicken lymphoma DT40 cells are deficient in G2 check-point in response to DNA damage and are defective in both DNA replication and cell cycle [34]. Collectively, these studies indicate that Chk1 is master regulator of cell cycle, cell survival and embryogenesis.
M. Patil et al.
In unperturbed cell cycle, Chk1 regulates DNA replication in S phase, G2/M transition or mitotic entry, and spindle checkpoint in M phase (Fig. 3). In S phase, Chk1 arrests cell cycle for DNA replication mainly by inducing Cdc25A degradation, resulting in the inhibition of CDK2. In U2OS cells, Chk1 phosphorylates Cdc25A at multiple sites and inhibition of Chk1 results in a marked accumulation of Cdc25A, suggesting that phosphorylation by Chk1 targets Cdc25A for degradation [35–37]. Consistently, conditional deletion of Chk1 from mammary cells in mice results in Cdc25A accumulation, which is accompanied by increases in S phase cells, accumulation of DNA damage, premature mitotic entry, and cell death [38]. Knockdown and inhibi-tion of Chk1 lead to increased initiation of DNA synthe-sis, increased single-strand DNA, aberrant fork structures, accumulation of double-strand breaks, DDR, and increased phosphorylation of ATR targets [33, 39]. These studies sug-gest that Chk1 may suppress late initiation of replication to prevent DNA damage for normal progression of S phase. However, the ATR/Chk1 pathway can also sense the strand stability and progression and initiate late replication to repair DNA breaks [40]. Collectively, all these observations suggest that Chk1 regulates replication checkpoint and is required for normal S phase progression and cell survival.
Mitotic entry is promoted by the activation of the cyclin B-CDK1 complex, which also involves the regulation by Chk1 [41]. Briefly, in late G2-phase, Chk1 accumulates at centrosome to phosphorylate Cdc25B (the phosphatase and activator of CDK1), resulting in the inhibition of Cdc25B and consequent suppression of CDK1. This regulation is critical to the prevention of premature mitotic entry in unperturbed cell cycle [41].
Finally, Chk1 has a critical role in spindle checkpoint in M-phase of the cell cycle. In support of this conclusion, Chk1-deficient chicken lymphoma DT 40 cells are viable, but they have increased levels of chromosome mis-segre-gation and genomic instability and fail to arrest in mitosis when treated with Taxol, a microtubule-stabilizing agent [42]. Similarly, Chk1 haploinsufficient primary mammary epithelial cells also exhibit misaligned chromosomes, chromosome missegregation, and enhanced binucleation [43]. Checkpoint kinase 1 knockdown U2OS cells undergo aberrant mitosis and fail to activate spindle checkpoint in response to Nocodazole treatment [37]. These studies suggest defective spindle checkpoint in the absence of Chk1. Mechanistically, Chk1 regulates spindle checkpoint through the activation and localization of Aurora B kinase, a regulator of spindle checkpoint that recruits BubR1 and MAD1 to the kinetochores [42, 43] to prevent APC/C acti-vation and delay anaphase onset [44]. Chk1 deficiency leads to mislocalization of Aurora B, BubR1 and MAD1 proteins, resulting in spindle checkpoint failure [42, 43] (Fig. 3).
Chk1 in DNA damage response
Originally identified in yeast, Chk1 is now recognized as an important mediator and signal transducer in DDR in other eukaryotes including mammals. In response to DNA damage, Chk1 is rapidly phosphorylated at serine-317 and serine-345 by ATR and becomes highly activated, resulting in the activation of DNA damage checkpoints [28, 45, 46]. Activated Chk1 phosphorylates several downstream targets to bring about cell cycle arrest, activate DNA repair path-ways, and induce apoptosis when DNA damage is severe [8, 47–49].
Chk1-induced cell cycle arrest in DDR
Checkpoint kinase 1 induces cell cycle arrest in DDR mainly by phosphorylating Cdc25 family phosphatases, wee1 kinase and controlling PLK1 (Fig. 4). The Cdc25 family is dual-specificity phosphatases that can dephospho-rylate Cdks in their ATP-binding loop, resulting in the acti-vation of Cdks and cell cycle progression. In mammalian
cells, there are three (A, B and C) isoforms of Cdc25, all
Fig. 3 Cell cycle checkpoints regulated by Chk1. Chk1 helps main-tain genomic integrity by regulating DNA replication, G2/M, and spindle checkpoints. Chk1 monitors DNA replication, slows down the replication to favor fork stability, prevent stalling of forks and DNA breaks. Chk1 prevents premature entry into mitosis before DNA replication is completed with high fidelity by inhibiting Cdc25 fam-ily phosphatases. Chk1 also regulates segregation of chromosomes through activation of aurora kinase to prevent genomic instability
Chk1 in DNA damage and cell cycle
of which can be phosphorylated by Chk1 in DDR. Interest-ingly, upon phosphorylation Cdc25A, B and C may employ distinct mechanisms to arrest the cell cycle [50, 51] (Fig. 5). For Cdc25A, phosphorylation by Chk1 targets it for protea-somal degradation, which leads to the inhibition of Cdk1 and Cdk2 resulting in cell cycle arrest at G1/S transition, S
phase, and G2/M transition [52, 53]. Interestingly, besides direct phosphorylation, Chk1 can also activate Nek11, which in turn phosphorylates Cdc25A to induce cell cycle arrest during DDR [54]. For Cdc25C, a main regulation seems to be its cellular localization. Specifically, phosphorylation of Cdc25C by Chk1 promotes its binding to 14-3-3 proteins leading to the sequestration of Cdc25C from Cdk1, Cdk1 inactivation and G2 arrest [4]. Cdc25B regulation by Chk1 seems to occur at centrosomes, where Chk1 phosphorylates Cdc25B leading to its sequestration from centrosome and inhibition of centrosomal Cdk1 [41].
In addition to Cdc25 phosphotases, Chk1 phosphoryl-ates wee1, the protein kinase that is responsible for the inhibitory phosphorylation of Cdk1 (Fig. 4). As a result, in response to DNA damage, the phosphorylation and activa-tion of wee1 by Chk1 leads to the inhibition of Cdk1 and cell cycle arrest at G2 phase [55, 56]. Chk1 is also a nega-tive regulator of PLK1 (polo like kinase 1), a mitotic kinase involved in centrosome maturation, spindle formation, and cytokinesis [57]. PLK1 phosphorylates wee1 and targets it for degradation, leading to Cdk1 activation and mitotic entry (Fig. 4). In addition, recent work suggests that, in response to DNA damage, Chk1 dissociates from the chro-matin leading to decreased phosphorylation and acetyla-tion of histone H3, which may contribute to DNA damage-associated transcriptional repression [58]. Interestingly, the repressed genes include cyclin B and Cdk1, although the role of the transcriptional repression in cell cycle arrest remains unclear.
Chk1-mediated DNA repair during DDR
As discussed above, Chk1 induces checkpoint activa-tion resulting in cell cycle arrest. In addition, in S-phase, Chk1 may slow down DNA replication by blocking the ini-tiation factor CDC45 in a Cdk2-independent manner [59]. Together, these mechanisms provide time for DNA repair to maintain genomic integrity in response to DNA damage. Notably, emerging evidence from the past few years has suggested a more direct role of Chk1 in DNA repair (Fig. 6).In this regard, Chk1 induces post-translational modifica-tion and activation of several important DNA repair factors. (1) Proliferating cell nuclear antigen (PCNA). One of the mechanisms of Chk1-mediated DNA repair is by inducing monoubiquitination of PCNA [60]. Upon ubiquitination, PCNA can activate translesion synthesis, a process that allows the replication machinery to pass or tolerate DNA lesions for replication. Specifically, at DNA damage sites, monoubiquitinated PCNA recruits specific translesion syn-thesis DNA polymerases to replace classical polymerases [61, 62], which can bypass or repair the damaged sites (lesion) for DNA replication [63]. (2) FANCe. In the repair
of DNA cross-links, Chk1 phosphorylates the FANCe
Fig. 4 Chk1 regulation of cell cycle progression. Chk1 regulates pro-gression of cell cycle by inhibiting Cdc25 family phosphatases and polo-like kinase 1(PLK1), and activating wee 1 kinase. Cdc25 is a phosphatase and activator of CDK1, whereas wee1 inhibits CDK1 by phosphorylation. PLK1 can activate CDK1 by inhibiting wee1.
PLK1 can also directly promote cell cycle progression
Fig. 5 Chk1-mediated cell cycle arrest in response to DNA dam-age. Chk1 phosphorylates Cdc25 family resulting in their inhibition. Chk1 can also activate Nek11, which further phosphorylates Cdc25A on multiple sites to target it for degradation. Cdc25A is crucial for G1-S transition, S phase progression, and mitotic entry; as a result, Cdc25A inhibition leads to G1 arrest, slows down replication, and G2 arrest. Chk1 phosphorylates Cdc25B to promote its binding to 14-3-3 protein and sequestration from centrosome. Chk1 also phosphorylates Cdc25C to induce its binding to 14-3-3 protein and nuclear exclusion. Cdc25B and Cdc25C inhibition mainly causes G2 arrest
M. Patil et al.
subunit of the Fanconi anemia (FA) core complex on two conserved sites serine-346 and serine-S374, resulting in its co-localization with FANCD2 at nuclear foci or DNA dam-age sites [44, 64]. Together with BRCA1 and 2, FANCe and ubiquitinated FANCD2 help resolve interstrand DNA crosslinks [44]. (3) Rad51. Checkpoint kinase 1 participates in homologous recombination repair by regulating Rad51. Knockdown or inhibition of Chk1 leads to accumulation of double-strand breaks and increased cell death in response to double-strand breaks induced by hydroxyurea and camp-tothecin. Mechanistically, Chk1 phosphorylates Rad51 at serine-309 and recruits it to DNA repair foci to promote homologous recombination repair and cell survival [65]. (4) TLK. In response to double-strand DNA breaks, Chk1 can also phosphorylate the serine-695 site of TLK (tousled-like kinase), an evolutionarily conserved serine/threonine kinase that is involved in chromatin assembly, DNA replica-tion, and repair. Interestingly, the phosphorylation of TLK by Chk1 depends on ATM [66]. Finally, it is noteworthy that Chk1 has also been suggested to stabilize replication forks and/or restart stalled replication forks; however, the under-lying mechanisms remain unclear.Chk1-induced cell death in DDR
There are few reports implicating Chk1 in apoptosis or cell death. Chk1 phosphorylates p73 at serine-47, resulting in a
drastic increase in p73 expression and transcription activity in response to DNA damage. Although p73 is a p53-related protein, it induces apoptosis independently of p53 [67, 68]. In contrast, there are instances where Chk1 inhibits apopto-sis through ATM/ATR-caspase2 and RPA-caspase3 pathway [69, 70]. Therefore, it remains unclear whether (and to what extent) Chk1 contributes to apoptosis and its regulation.
Regulation of Chk1
At the sequence level, Chk1 is highly conserved from yeast to humans [52] (Fig. 7
). Structurally, Chk1 has a conserved N-terminal kinase domain, a linker region, a regulatory SQ/TQ domain, and a C-terminal domain with unclear func-tions [52, 71]. It is noteworthy that Chk1 and Chk2 are not homologous in sequence, although they both have check-point function and share some phosphorylation substrate proteins.
It is well recognized that Chk1 is activated upon phospho-rylation at two conserved sites, serine-317 and serine-345 [28, 46, 72]. Although phosphorylation at these two sites is indispensable for the function of Chk1, it remains elu-sive as to how exactly the phosphorylation activates Chk1 [45]. The study of crystallographic structure revealed that in the absence of phosphorylation, the Chk1 kinase domain assumes an open, active conformation [73]. Indeed, the
Fig. 6 Chk1-mediated DNA repair. Activated Chk1 phosphorylates several substrates to mediate DNA repair and apoptosis. Chk1 slows down DNA replication directly by affecting CDC45 loading on replica-tion complex to allow time for DNA repair. Chk1 promotes monoubiq-uitination of PCNA, which recruits translesion polymerases to help translesion DNA synthesis. Chk1 phosphorylates TLK-tousled like kinase to promote chromatin assembly. Chk1 phosphorylates Rad51 to target it to double-strand breaks to promote HRR-homologous
recombination and repair. Chk1 phosphorylates FANCe and mon-oubiquitinates FANCD2, which help resolve ICR-interstrand cross-link DNA lesion. Chk1 also regulates replication fork stability, fork
restart, and late origins of replication, the mechanistic details of which are currently unclear. In addition to DNA repair, Chk1 may also induce cell cycle arrest and apoptosis by regulating different sub-strates
Chk1 in DNA damage and cell cycle kinase domain has significantly higher kinase activity when compared to full-length Chk1. These observations indicate that Chk1 kinase domain has a constitutively active confor-mation that does not rely on phosphorylation for activation. One model of Chk1 activation proposes that normally the C-terminal domain interacts with the kinase domain to mask the active site and the phosphorylation at serine-317 and ser-ine-345 dissociates these two domains leading to Chk1 acti-vation. This model is supported by several lines of evidence. First, C-terminal truncation mutants show higher catalytic activity than intact Chk1 [73]. Second, in binding assays, ectopically expressed C-terminal domain of rat Chk1 binds to the N-terminal kinase domain [74]. In addition, in Xeno-pus egg extracts, ectopically expressed C-terminal domain can interact with N-terminal Chk1 domain and inhibit its activity but not full-length Chk1 [71]. The inhibitory effect of C-terminal Chk1 domain on N-terminal kinase domain can be overcome by treatment with aphidicolin and phos-phomimetic mutations of ATR [71], suggesting that the C-terminal domain indeed inhibits kinase activity. Together, these observations suggest that the C-terminal domain is inhibitory to the kinase domain in Chk1 and, upon phos-phorylation, this inhibition is disabled. while this “intra-molecular inhibition” model is supported by several lines of evidence, it has been challenged recently. For exam-ple, several C-terminal truncation mutants lead to loss of function in Chk1 [45, 75], suggesting that removal of the C-terminal domain is not sufficient for Chk1 activation and Chk1 regulation may be more complex than “intramolecular
inhibition”. In addition to serine-317 and serine-345, Chk1
Fig. 7 CHk1 sequence align-ment between human, mice, fruit fly, Xenopus and yeast. Chk1 sequences of human, mice, Drosophila , Xenopus , and yeast were analyzed using T-coffee, a web server for mul-tiple sequence alignment tool. The N-terminal kinase domain and regulatory SQ/TQ domain of Chk1 are highly conserved, while the C-terminal domain is less conserved. Asterisk indicates highly conserved sequence, Colon indicates con-served substitution, Dot indicate semi conserved substitutions
M. Patil et al.
has also been reported to have autophosphorylation at ser-ine-296, which plays a role in G2 checkpoint [76]. Interest-ingly, the autophosphorylation is consequent to phosphoryl-ation at serine-317 and serine-345 through intramolecular mechanisms. It is noteworthy that serine-296 phosphoryla-tion does not affect the kinase activity of Chk1 [77].
Interestingly, a recent study by walker and colleagues indicates that Chk1 may be subjected to a new mechanism of regulation, called “de-repression”. In this model, Chk1 is antagonized by an inhibitory or repressing factor(s), the dissociation of which leads to Chk1 activation. In support of this possibility, Chk1 immunoprecipitated from untreated cells demonstrates significantly higher kinase activity after being washed with a more stringent buffer. Notably, Chk1 immunoprecipitated from DNA damaged (by aphidicolin) cells also shows significantly higher kinase activity when washed with the stringent buffer, although the increase is somewhat smaller than that of untreated cells. In addition, stringent buffer washes also increase the kinase activity of Chk1 (S345A) mutant from transfected cells [78], suggest-ing that phosphorylation at serine-345 is not obligatory for Chk1 kinase activity per se, although it plays a role in Chk1
activation in cells. Furthermore, Chk1 can be activated in a claspin-dependent manner in response to genotoxic stress independently of ATM/ATR and associated phosphoryla-tion [79]. Together, these studies support the possibility of additional regulatory mechanisms for Chk1, in addition to phosphorylation. The “de-repression” model is intriguing and tantalizing, but the identity of the “repressing” factor(s) of Chk1 is not known.
Our latest work has identified a splice variant of Chk1, which may act as an endogenous repressor of Chk1 [80]. This variant is spliced in a way that the exon 3 of Chk1 is deleted, leading to the production of an N-terminally trun-cated protein, which we call Chk1-short or Chk1-S. Due to the truncation, Chk1-S lacks part of the kinase domain including the ATP-binding (Fig. 8a) and, as expected, does not possess protein kinase activity. Importantly, a series of in vitro and in vivo tests showed that Chk1-S directly interacts with Chk1 at its N-terminal domain and inhibits Chk1 kinase activity. In addition, we confirmed the find-ing by walker and colleagues that stringent buffer washes can increase the activity of Chk1 immunoprecipitated from
cells. Moreover, we showed that the addition of exogenous
Fig. 8 Physical and functional interactions between Chk1 and Chk1-S. a Schematic representation of Chk1 and Chk1S domains and their interaction. Checkpoint kinase 1 has ATP binding domain, N-terminal kinase domain, SQ/TQ domain, and C-terminal domain. Chk1S lacks ATP binding domain. C-terminal domain of Chk1 inter-acts with N-terminal domain of CHK1-S. b Regulation of Chk1 by Chk1-S. Chk1-S interacts with Chk1 to inhibit its kinase activity. In unperturbed cell cycles, Chk1-S is expressed in late S to G2 phase to inhibit Chk1 and promote mitotic entry. In DNA damage, Chk1 is phosphorylated to prevent Chk1-S binding and inhibition, result-ing in Chk1 activation. when Chk1-S is overexpressed, it antagonizes Chk1, leading to premature mitotic entry and consequent cell death via mitotic catastrophe. Figure 8b is adopted from Pabla et al. PNAS 109, 197-202, 2012
Chk1 in DNA damage and cell cycle
Chk1-S can reverse the effect of stringent buffer washing. Together, these results suggest that Chk1-S may be one of the key endogenous repressing factors of Chk1. In an unper-turbed cell cycle, Chk1-S expression increases significantly in late G2 and early M phases coinciding with the decrease in Chk1 activity for mitotic entry. Overexpression of Chk1-S leads to premature mitotic entry followed by cell death in the form of mitotic catastrophe. These observations sup-port an important role of Chk1-S in the regulation of normal cell cycles by binding and antagonizing Chk1. In response to DNA damage, the interaction between Chk1 and Chk1-S is lost in an ATR-dependent manner (Fig. 8b). Remarkably, mutation of Chk1 at serine-317 and serine-345 prevents the dissociation of Chk1 from Chk1-S during DNA dam-age, indicating that the dissociation of Chk1 from Chk1-S in DDR depends on Chk1 phosphorylation. Based on these observations, we propose that Chk1-S is a key regulator of Chk1 in both normal cell cycles and DDR. Mechanisti-cally, Chk1-S binds to Chk1 to act as a repressor to block Chk1 activity. In normal cell cycles, Chk1-S is drastically increased at late G2 phase to repress Chk1 to promote mitotic entry. In DDR, ATR-mediated Chk1 phosphoryla-tion at serine-317 and serine-345 leads to the dissociation of Chk1-S from Chk1, resulting in Chk1 activation and cell cycle arrest (Fig. 8b).
In addition to the above-described regulatory mecha-nisms, Chk1 is also known to be subjected by proteasomal degradation. Interestingly, proteasomal degradation of Chk1 may depend on its phosphorylation status. In camptothecin-induced DNA damage, Chk1 phosphorylation at serine-345 targets the protein for proteosomal degradation [81]. It was later shown that the phosphorylation exposes a dragon-like region at the C-terminus, which is recognized by the F-box protein called Fbx6, leading to Chk1 ubiquitination and proteasomal degradation and termination of the checkpoint [82]. Consistently, there is an inverse correlation between Chk1 and Fbx6 expression in cultured cancer cells and breast cancer tissues. In addition, overexpression of Fbx 6 leads to degradation of chk1 and increased cellular sensitiv-ity to camptothecin [82].
Chk1 substrates
Despite the multiple roles of Chk1 in normal cell cycles and DDR, only a few phosphorylation substrates of Chk1 have been identified thus far. As a result, identification of func-tional substrates of Chk1 is currently a subject of intense investigation. The consensus sequence of Chk1-specific sub-strates has been suggested by analyzing the peptide derived from Xenopus. Cdc25C containing serine-287, a typical Chk1-phosphorylation site. Indeed, the consensus sequence has been useful for predicting Chk1 substrates including PDS1 (an anaphase inhibitor) and wee1 [83]. Nonetheless, the consensus sequence is insufficient to explain other Chk1 substrates. Some of the Chk1 substrates may be context-dependent and do not rely on the consensus sequence for phosphorylation. In this regard, a substrate may be phospho-rylated by Chk1 through interaction with adaptor proteins.
A recent study has conducted a global phospho-proteomic screen using analogue-sensitive Chk1 and suggested 171 proteins as its potential phosphorylation substrates. Moreo-ver, KAP1, a protein phosphorylated in response to DNA damage, has been validated to be a Chk1 substrate in both in vitro and in vivo studies and KAP1 phosphorylation can be used as a read out for Chk1 activation [47]. However, most of the substrates implied in this study remain to be validated. It is expected that new and important functional substrates of Chk1 will be discovered in the next few years to provide new insights into the multiple functions of Chk1 in the cell cycle and DDR.
Chk1 in cancer
Checkpoint kinase 1 mutations or loss are very rare in can-cer. A logical explanation is that Chk1 plays an essential role in cell cycle regulation, cell proliferation and survival [28, 29, 80], and as a result, cells with defective Chk1 are eliminated during tumorigenesis. Nonetheless, par-tial ablation of Chk1 (heterozygous) favors tumor forma-tion in wNT1 oncogenic mice [28]. In addition, loss-of-function Chk1 mutations have been reported in stomach, endometrial, and colorectal cancers [84–87]. Mutations have been mapped to the microsatellite instability region in the coding region of Chk1 with a stretch of nine adenine nucleotides [86]. Frame-shift mutations lead to the forma-tion of a truncated protein in colorectal and endometrial cancers lacking part of the kinase domain and the C-ter-minal domain [86]. Intriguingly, in these cancers is that the second allele is normal, resulting in the expression of functional Chk1 albeit at a lower level than that of normal tissues [88]. Monoallelic mutations leading to partial loss of expression are also observed in ATR in endometrial and stomach cancers [85]. These studies support the haploin-sufficient tumor model in which monoallelic mutations in several genes of the same pathway favor tumorigenesis [88]. In mice, conditional deletion of Chk1 in mammary epithelial cells fails to produce tumors; instead it leads to cell death [38]. However, the mice haploinsufficient for both Chk1 and p53 develop tumors in mammary glands [89]. Similarly, complete loss of Chk1 in skin suppresses chemically induced carcinogenesis, whereas partial (hap-loinsufficient) deletion of Chk1 promotes benign malig-nant tumor progression [90]. Triple-negative (estrogen-/ progesterone-/HeR2-) breast cancers express a very high
M. Patil et al.
level of Chk1 and have poor clinical outcomes, suggest-ing that Chk1 favors cell proliferation [91]. Together, these studies indicate that Chk1 is essential for cell survival and its haploinsufficiency may promote cancer, especially in the presence of mutations of other relevant genes. Of note, Chk1-S, the splice variant and endogenous inhibitor of Chk1, is expressed at significantly higher levels in cancer tissues than normal tissues and notably, Chk1-S expres-sion correlates with the degree of malignancy in ovarian and testicular tumors [80]. Interestingly, Chk1 expression is also higher in cancer. It is suggested that relatively high levels of Chk1 and Chk1-S may favor cell proliferation in cancer.
Targeting Chk1 for cancer therapy
DNA damaging agents are the most commonly used drugs for cancer therapy. By damaging DNA, these chemother-apy drugs induce cell cycle arrest to prevent cell prolifera-tion and trigger cell death in cancers. Many of these agents induce G1 arrest in a p53-dependent manner and G2 arrest in a Chk1-dependent manner. G1 arrest is defective in many cancers since more than 50 % of cancers are defective in p53; under these situations, G2 arrest becomes a main path-way for cancer cell survival. Hence, Chk1-dependent G2 checkpoint is a major target for chemotherapy [92–95]. In this regard, Chk1 inhibition or knockdown increases the sensitivity of p53-deficient cancer cells to DNA damage agents [33, 96]. However, it has also been reported in certain cell lines that Chk1 is essential for G2 checkpoint irrespec-tive of the p53 status and hence Chk1 inhibition may not preferantially kill p53-deficient cells [97]. It has also been shown that Chk1 inhibition increases the sensitivity of can-cer cells to anti-mitotic agents [98, 99]. In addition, Chk1 is a client of HSP90 and inhibition of HSP90 leads to the loss of Chk1 resulting in the sensitization of cancer cells to Gemcitabine, an S phase chemotherapeutic agent [100]. Chk1 and wee1 kinase inhibitors in combination have more than additive effect on different cancer cell line prolifera-tion and human xenograft models [101]. Therefore, Chk1 inhibitors may target multiple cell cycle phases to override the checkpoint responses during cancer therapy, leading to increased therapeutic efficacy. Currently, Chk1 inhibitors are being developed and tested alone or in combination with DNA damaging agents in clinical trials for cancer therapy [8, 36]. However, so far, none of these have passed phase III clinical trials. Obviously, the specificity and potency of Chk1 inhibitors are critical to their clinical use. In addition, their side effects have to be carefully evaluated because Chk1 has an important role in cell cycle regulation and its inhibition may adversely affect cell viability and function in normal tissues.Concluding remarks
Checkpoint kinase 1 plays an essential role in cell cycle regulation and DNA damage response. In unperturbed cell cycle, Chk1 regulates G1/S transition, S phase, mitotic entry, and mitosis. In DDR, Chk1 is an important signal transducer and the trigger of G2 checkpoint activation. The role of Chk1 in unperturbed cell cycle and tissue physiol-ogy is only beginning to be understood. Chk1 regulates S phase progression by controlling DNA replication, fork sta-bility and late origins of replication the mechanistic details of which are yet to be identified. The role of Chk1 in the development and pathogenesis in different tissues are yet to be investigated. In addition, although Chk1 regulates sev-eral checkpoints, only a few Chk1 substrate proteins have been identified. The discovery of Chk1-S as an important regulator of Chk1 in both unperturbed cell cycle and DDR has opened new areas of investigation. Finally, Chk1 may be an effective therapeutic target in diseases. In this regard, Chk1 inhibitors, when used together with other therapeutic agents, may significantly enhance the chemotherapy effi-cacy in cancer treatment.
References
1. walworth N, Davey S, Beach D (1993) Fission yeast Chk1-pro-
tein kinase links the rad checkpoint pathway to Cdc2. Nature 363:368–371
2. Kumagai A, Guo Z, emami KH, wang SX, Dunphy wG (1998)
The Xenopus Chk1 protein kinase mediates a caffeine-sensitive
pathway of checkpoint control in cell-free extracts. J Cell Biol 142:1559–1569
3. Fogarty P, Kalpin RF, Sullivan w (1994) The Drosophila mater-
nal-effect mutation grapes causes a metaphase arrest at nuclear cycle 13. Development 120:2131–2142
4. Peng CY, Graves PR, Thoma RS, wu Z, Shaw AS, Piwnica-
worms H (1997) Mitotic and G2 checkpoint control: regulation
of 14–3-3 protein binding by phosphorylation of Cdc25C on ser-
ine-216. Science 277:1501–1505
5. elledge SJ (1996) Cell cycle checkpoints: preventing an identity
crisis. Science 274:1664–1672
6. Nurse P (2000) A long twentieth century of the cell cycle and
beyond. Cell 100:71–78
7. Cimprich KA, Cortez D (2008) ATR: an essential regulator of
genome integrity. Nat Rev Mol Cell Biol 9:616–627
8. Dai Y, Grant S (2010) New insights into checkpoint kinase 1 in
the DNA damage response signaling network. Clin Cancer Res 16:376–383
9. Bartek J, Lukas J (2007) DNA damage checkpoints: from initia-
tion to recovery or adaptation. Curr Opin Cell Biol 19:238–245 10. Lee JH, Paull TT (2005) ATM activation by DNA double-strand
breaks through the Mre11-Rad50-Nbs1 complex. Science 308:551–554
11. Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM
through intermolecular autophosphorylation and dimer dissocia-
tion. Nature 421:499–506
12. Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S (2004)
Molecular mechanisms of mammalian DNA repair and the DNA
damage checkpoints. Annu Rev Biochem 73:39–85
Chk1 in DNA damage and cell cycle
13. Nam eA, Cortez D (2011) ATR signalling: more than meeting at
the fork. Biochem J 436:527–536
14. Zou L, elledge SJ (2003) Sensing DNA damage through ATRIP
recognition of RPA-ssDNA complexes. Science 300:1542–1548 15. Cortez D, Guntuku S, Qin J, elledge SJ (2001) ATR and ATRIP:
partners in checkpoint signaling. Science 294:1713–1716
16. Parrilla-Castellar eR, Arlander SJ, Karnitz L (2004) Dial 9–1-1
for DNA damage: the Rad9-Hus1-Rad1 (9–1-1) clamp complex.
DNA Repair 3:1009–1014
17. Delacroix S, wagner JM, Kobayashi M, Yamamoto K, Kar-
nitz LM (2007) The Rad9-Hus1-Rad1 (9–1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev 21:1472–1477 18. Lee J, Kumagai A, Dunphy wG (2007) The Rad9-Hus1-Rad1
checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem 282:28036–28044
19. Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z (2011) hMSH2
recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem 286:10411–10418 20. Kumagai A, Dunphy wG (2000) Claspin, a novel protein required
for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell 6:839–849
21. Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jack-
son SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8:37–45 22. Stiff T, walker SA, Cerosaletti K, Goodarzi AA, Petermann e,
Concannon P, O’Driscoll M, Jeggo PA (2006) ATR-dependent phosphorylation and activation of ATM in response to Uv treat-
ment or replication fork stalling. eMBO J 25:5775–5782
23. Pabla N, Huang S, Mi QS, Daniel R, Dong Z (2008) ATR-Chk2
signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283:6572–6583
24. enders GH (2008) expanded roles for Chk1 in genome mainte-
nance. J Biol Chem 283:17749–17752
25. Sorensen CS, Syljuasen RG (2012) Safeguarding genome integ-
rity: the checkpoint kinases ATR, CHK1 and wee1 restrain CDK activity during normal DNA replication. Nucleic Acids Res 40:477–486
26. Brown eJ, Baltimore D (2000) ATR disruption leads to chromo-
somal fragmentation and early embryonic lethality. Genes Dev 14:397–402
27. de Klein A, Muijtjens M, van Os R, verhoeven Y, Smit B, Carr
AM, Lehmann AR, Hoeijmakers JH (2000) Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol 10:479–482
28. Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo
G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, elledge SJ (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint.
Genes Dev 14:1448–1459
29. Takai H, Tominaga K, Motoyama N, Minamishima YA, Naga-
hama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M (2000) Aberrant cell cycle checkpoint function and early embry-
onic death in Chk1(-/-) mice. Genes Dev 14:1439–1447
30. Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR,
Uy GL, Goldberg ML, Sullivan w (1997) The Drosophila grapes
gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol 7:418–426
31. Purdy A, Uyetake L, Cordeiro MG, Su TT (2005) Regulation of
mitosis in response to damaged or incompletely replicated DNA require different levels of Grapes (Drosophila Chk1). J Cell Sci 118:3305–3315
32. Royou A, McCusker D, Kellogg DR, Sullivan w (2008)
Grapes(Chk1) prevents nuclear CDK1 activation by delaying cyclin B nuclear accumulation. J Cell Biol 183:63–75
33. Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C,
Johansson F, Helleday T, Sehested M, Lukas J, Bartek J (2005)
Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage.
Mol Cell Biol 25:3553–3562
34. Zachos G (2003) Chk1-defcient tumour cells are viable but
exhibit multiple checkpoint and survival defects. eMBO Rep 22(3):713–723
35. Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand
L, Khanna KK, Zhou BB, Bartek J, Lukas J (2003) Chk1 regu-
lates the S phase checkpoint by coupling the physiological turn-
over and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3:247–258
36. Carrassa L, Damia G (2011) Unleashing Chk1 in cancer therapy.
Cell Cycle 10:2121–2128
37. Carrassa L, Sanchez Y, erba e, Damia G (2009) U2OS cells
lacking Chk1 undergo aberrant mitosis and fail to activate the spindle checkpoint. J Cell Mol Med 13:1565–1576
38. Lam MH, Liu Q, elledge SJ, Rosen JM (2004) Chk1 is haplo-
insufficient for multiple functions critical to tumor suppression.
Cancer Cell 6:45–59
39. Petermann e, woodcock M, Helleday T (2010) Chk1 promotes
replication fork progression by controlling replication initiation.
Proc Natl Acad Sci USA 107:16090–16095
40. Maya-Mendoza A (2007) Chk1 regulates the density of active
replication origins during the vertebrate S phase. eMBO Rep 26(11):2719–2731
41. Kramer A, Mailand N, Lukas C, Syljuasen RG, wilkinson CJ,
Nigg eA, Bartek J, Lukas J (2004) Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol 6:884–891
42. Zachos G, Black eJ, walker M, Scott MT, vagnarelli P, earn-
shaw wC, Gillespie DA (2007) Chk1 is required for spindle checkpoint function. Dev Cell 12:247–260
43. Peddibhotla S, Lam MH, Gonzalez-Rimbau M, Rosen JM
(2009) The DNA-damage effector checkpoint kinase 1 is essen-
tial for chromosome segregation and cytokinesis. Proc Natl Acad Sci USA 106:5159–5164
44. Guervilly JH, Mace-Aime G, Rosselli F (2008) Loss of CHK1
function impedes DNA damage-induced FANCD2 monoubiqui-
tination but normalizes the abnormal G2 arrest in Fanconi ane-
mia. Hum Mol Genet 17:679–689
45. Tapia-Alveal C, Calonge TM, O’Connell MJ (2009) Regulation
of chk1. Cell Div 4:8
46. Zhao H, Piwnica-worms H (2001) ATR-mediated checkpoint
pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 21:4129–4139
47. Blasius M, Forment Jv, Thakkar N, wagner SA, Choudhary
C, Jackson SP (2011) A phospho-proteomic screen identifies substrates of the checkpoint kinase Chk1. Genome Biol 12: R78
48. Harper Jw, elledge SJ (2007) The DNA damage response: ten
years after. Mol Cell 28:739–745
49. Chen Y, Poon RY (2008) The multiple checkpoint functions of
CHK1 and CHK2 in maintenance of genome stability. Front Biosci 13:5016–5029
50. Donzelli M, Draetta GF (2003) Regulating mammalian check-
points through Cdc25 inactivation. eMBO Rep 4:671–677
51. Uto K, Inoue D, Shimuta K, Nakajo N, Sagata N (2004) Chk1,
but not Chk2, inhibits Cdc25 phosphatases by a novel common mechanism. eMBO J 23:3386–3396
52. Sanchez Y, wong C, Thoma RS, Richman R, wu Z, Piwnica-
worms H, elledge SJ (1997) Conservation of the Chk1 check-
point pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497–1501
53. Mailand N, Falck J, Lukas C, Syljuasen RG, welcker M, Bar-
tek J, Lukas J (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science 288:1425–1429
M. Patil et al.
54. Melixetian M, Klein DK, Sorensen CS, Helin K (2009) NeK11
regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat Cell Biol 11:1247–1253
55. O’Connell MJ, Raleigh JM, verkade HM, Nurse P (1997) Chk1
is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. eMBO J 16:545–554
56. Lee J, Kumagai A, Dunphy wG (2001) Positive regulation
of wee1 by Chk1 and 14–3-3 proteins. Mol Biol Cell 12: 551–563
57. Tang J, erikson RL, Liu X (2006) Checkpoint kinase 1 (Chk1)
is required for mitotic progression through negative regula-
tion of polo-like kinase 1 (Plk1). Proc Natl Acad Sci USA 103:11964–11969
58. Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito
H, Nakanishi M (2008) Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression.
Cell 132:221–232
59. Liu P, Barkley LR, Day T, Bi X, Slater DM, Alexandrow MG,
Nasheuer HP, vaziri C (2006) The Chk1-mediated S-phase checkpoint targets initiation factor Cdc45 via a Cdc25A/Cdk2-
independent mechanism. J Biol Chem 281:30631–30644
60. Yang XH, Shiotani B, Classon M, Zou L (2008) Chk1 and Claspin
potentiate PCNA ubiquitination. Genes Dev 22:1147–1152
61. Kannouche PL, wing J, Lehmann AR (2004) Interaction of
human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14:491–500
62. Freudenthal BD, Gakhar L, Ramaswamy S, washington MT
(2010) Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nat Struct Mol Biol 17:479–484
63. Prakash S, Johnson Re, Prakash L (2005) eukaryotic translesion
synthesis DNA polymerases: specificity of structure and func-
tion. Annu Rev Biochem 74:317–353
64. wang X, Kennedy RD, Ray K, Stuckert P, ellenberger T,
D’Andrea AD (2007) Chk1-mediated phosphorylation of FANCe is required for the Fanconi anemia/BRCA pathway. Mol Cell Biol 27:3098–3108
65. Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lun-
din C, Bartek J, Helleday T (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombina-
tion repair. Nat Cell Biol 7:195–201
66. Groth A, Lukas J, Nigg eA, Sillje HH, wernstedt C, Bartek J,
Hansen K (2003) Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. eMBO J 22:1676–1687
67. Gonzalez S, Prives C, Cordon-Cardo C (2003) p73 alpha regu-
lation by Chk1 in response to DNA damage. Mol Cell Biol 23:8161–8171
68. Urist M, Tanaka T, Poyurovsky Mv, Prives C (2004) p73 induc-
tion after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev 18:3041–3054
69. Myers K, Gagou Me, Zuazua-villar P, Rodriguez R, Meuth M
(2009) ATR and Chk1 suppress a caspase-3-dependent apop-
totic response following DNA replication stress. PLoS Genet 5:e1000324
70. Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans
R, Pascual J, Imamura S, Kishi S, Amatruda JF, Kanki JP, Green DR, D’Andrea AA, Look AT (2008) Chk1 suppresses a cas-
pase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 133:864–877
71. Katsuragi Y, Sagata N (2004) Regulation of Chk1 kinase by
autoinhibition and ATR-mediated phosphorylation. Mol Biol Cell 15:1680–1689
72. Capasso H, Palermo C, wan S, Rao H, John UP, O’Connell
MJ, walworth NC (2002) Phosphorylation activates Chk1 and
is required for checkpoint-mediated cell cycle arrest. J Cell Sci 115:4555–4564
73. Chen P, Luo C, Deng Y, Ryan K, Register J, Margosiak S, Temp-
czyk-Russell A, Nguyen B, Myers P, Lundgren K, Kan CC, O’Connor PM (2000) The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation.
Cell 100:681–692
74. Shann YJ, Hsu MT (2001) Cloning and characterization of
liver-specific isoform of Chk1 gene from rat. J Biol Chem 276:48863–48870
75. Kosoy A, O’Connell MJ (2008) Regulation of Chk1 by its C-ter-
minal domain. Mol Biol Cell 19:4546–4553
76. Okita N, Minato S, Ohmi e, Tanuma S, Higami Y (2012) DNA
damage-induced CHK1 autophosphorylation at Ser296 is regu-
lated by an intramolecular mechanism. FeBS Lett 586:3974–3979 77. Kasahara K, Goto H, enomoto M, Tomono Y, Kiyono T, Ina-
gaki M (2010) 14–3-3gamma mediates Cdc25A proteolysis to block premature mitotic entry after DNA damage. eMBO J 29:2802–2812
78. walker M, Black eJ, Oehler v, Gillespie DA, Scott MT (2009)
Chk1 C-terminal regulatory phosphorylation mediates check-
point activation by de-repression of Chk1 catalytic activity.
Oncogene 28:2314–2323
79. Rodriguez-Bravo v, Guaita-esteruelas S, Florensa R, Bachs O,
Agell N (2006) Chk1- and claspin-dependent but ATR/ATM- and Rad17-independent DNA replication checkpoint response in HeLa cells. Cancer Res 66:8672–8679
80. Pabla N, Bhatt K, Dong Z (2011) Checkpoint kinase 1 (Chk1)-
short is a splice variant and endogenous inhibitor of Chk1 that regulates cell cycle and DNA damage checkpoints. Proc Natl Acad Sci 109:197–202
81. Zhang Yw, Otterness DM, Chiang GG, Xie w, Liu YC, Mercu-
rio F, Abraham RT (2005) Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell 19:607–618
82. Zhang Yw, Brognard J, Coughlin C, You Z, Dolled-Filhart M,
Aslanian A, Manning G, Abraham RT, Hunter T (2009) The F box protein Fbx6 regulates Chk1 stability and cellular sensitivity to replication stress. Mol Cell 35:442–453
83. Hutchins JR, Hughes M, Clarke PR (2000) Substrate specificity
determinants of the checkpoint protein kinase Chk1. FeBS Lett 466:91–95
84. Bertoni F, Codegoni AM, Furlan D, Tibiletti MG, Capella C,
Broggini M (1999) CHK1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Gene Chromosome Canc 26:176–180
85. Menoyo A (2001) Somatic mutations in the DNA damage-
response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res 61(21):7727–7730
86. Codegoni AM, Bertoni F, Colella G, Caspani G, Grassi L,
D’Incalci M, Broggini M (1999) Microsatellite instability and frameshift mutations in genes involved in cell cycle progression or apoptosis in ovarian cancer. Oncol Res 11:297–301
87. vassileva v (2002) Genes involved in DNA repair are mutational
targets in endometrial cancers with microsatellite instability.
Cancer Res 62(14):4095–4099
88. Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint
control and cancer. Canc Cell 3:421–429
89. Fishler T, Li YY, wang RH, Kim HS, Sengupta K, vassilopoulos
A, Lahusen T, Xu X, Lee MH, Liu Q, elledge SJ, Ried T, Deng CX (2010) Genetic instability and mammary tumor formation in mice carrying mammary-specific disruption of Chk1 and p53.
Oncogene 29:4007–4017
90. Tho LM, Libertini S, Rampling R, Sansom O, Gillespie DA
(2012) Chk1 is essential for chemical carcinogen-induced mouse skin tumorigenesis. Oncogene 31:1366–1375
Chk1 in DNA damage and cell cycle
91. verlinden L, vanden Bempt I, eelen G, Drijkoningen M, verlin-
den I, Marchal K, De wolf-Peeters C, Christiaens MR, Michiels
L, Bouillon R, verstuyf A (2007) The e2F-regulated gene Chk1 is highly expressed in triple-negative estrogen receptor/
progesterone receptor/HeR-2 breast carcinomas. Cancer Res 67:6574–6581
92. Zhou BB, Anderson HJ, Roberge M (2003) Targeting DNA
checkpoint kinases in cancer therapy. Cancer Biol Ther 2:S16–S22
93. Zhou BB, Bartek J (2004) Targeting the checkpoint kinases:
chemosensitization versus chemoprotection. Nat Rev Cancer 4:216–225
94. Lieberman HB (2008) DNA damage repair and response pro-
teins as targets for cancer therapy. Curr Med Chem 15:360–367 95. Tenzer A, Pruschy M (2003) Potentiation of DNA-damage-
induced cytotoxicity by G2 checkpoint abrogators. Curr Med Chem Anticancer Agents 3:35–46
96. Koniaras K (2001) Inhibition of Chk1-dependent G2 DNA dam-
age checkpoint radiosensitizes p53 mutant human cells. Onco-
gene 20(51):7453–7463
97. Zenvirt S, Kravchenko-Balasha N, Levitzki A (2010) Status of
p53 in human cancer cells does not predict efficacy of CHK1 kinase inhibitors combined with chemotherapeutic agents.
Oncogene 29:6149–6159
98. Blagosklonny Mv (2002) Sequential activation and inactivation
of G2 checkpoints for selective killing of p53-deficient cells by microtubule-active drugs. Oncogene 21:6249–6254
99. Xiao Z, Xue J, Semizarov D, Sowin TJ, Rosenberg SH, Zhang
H (2005) Novel indication for cancer therapy: Chk1 inhibition
sensitizes tumor cells to antimitotics. Int J Cancer 115:528–538 100. Arlander SJ, eapen AK, vroman BT, McDonald RJ, Toft DO, Karnitz LM (2003) Hsp90 inhibition depletes Chk1 and sensitizes
tumor cells to replication stress. J Biol Chem 278:52572–52577 101. Guertin AD, Martin MM, Roberts B, Hurd M, Qu X, Miselis NR, Liu Y, Li J, Feldman I, Benita Y, Bloecher A, Toniatti C, Shumway SD (2012) Unique functions of CHK1 and wee1 underlie synergistic anti-tumor activity upon pharmacologic inhibition. Cancer cell Int 12:45
滑坡稳定性计算书
第一部分参数选取 根据钻探揭露,滑带土为黄褐色粉质亚粘土夹少量砂板岩角砾,位于人工堆积层与下层基岩之间,深度在2-7m不等,厚约0.2-0.3m,断面光滑。 2、滑带土参数的取值 (1)参数反演 滑坡中的滑带土为基覆交界面的亚粘土层,由于野外取样时,所取滑带土样为已经扰动过的土样,因此在进行岩土试验参数统计及经验类比的取值时,滑带土的C、φ值采用滑坡在暴雨工况下,取稳定系数为1.03时反演取值,其反演计算模型,选定H1滑坡的2-2’剖面。反演计算剖面及内容见计算书。 采用反演公式和 经反演,滑坡滑带土在暴雨条件下C、φ值见下表。 (2)工程类比经验:借鉴蜀通公司对H2滑坡所做的勘查工作,天然条件下C 值为6.7KPa,φ为18.5°,暴雨条件下C值为3.3-4.6KPa,φ为12.3°。 (3)试验值: (4)综合取值: 根据滑带土的试验、剖面反演及工程类比的结果,滑带土而天然工况下的取值主要依据试验结果,在暴雨工况下参数取值主要采取加权平均,对试验值、反演值和工程类比值采取加权平均方法从而得出暴雨工况下的滑带土的c、φ值。目前各滑坡处于蠕动变形阶段,因此对试验值取较高的权重。三种取值的权重分别是0.5、0.3、0.2。据此得出暴雨工况下的滑带土的参数值。 滑带土参数取值为天然重度为19.0 kN/m3,饱和重度为20.5kN/m3,天然条件下C值为7.0KPa,φ为18.5°;饱和条件下c值为3.8KPa,φ为13.0°。 一、2-2’反演 滑坡剩余下滑力计算 计算项目: 2-2暴雨 ===================================================================== 原始条件: 滑动体重度= 19.000(kN/m3) 滑动体饱和重度= 20.500(kN/m3) 安全系数= 1.030 不考虑动水压力和浮托力 不考虑承压水的浮托力 不考虑坡面外的静水压力的作用 不考虑地震力 坡面线段数: 41, 起始点标高 0.000(m) 段号投影Dx(m) 投影Dy(m) 附加力数 1 0.144 0.351 0 2 0.386 1.579 0 3 0.279 0.673 0 4 0.541 0.977 0 5 0.232 0.793 0 6 0.601 0.846 0 7 0.475 0.781 0 8 0.266 0.496 0 9 0.353 0.812 0 10 0.518 0.658 0 11 0.110 0.265 0 12 0.102 0.204 0 13 0.197 0.490 0 14 0.234 0.464 0 15 0.197 0.147 0
CheckPoint 防火墙 双机 HA 实施 方案
本文由no1moonboy贡献 doc1。 双 CheckPoint 防火墙实施方案 目 录 第一章 客户环境概述…… 4 1.1 概述 …… 4 1.2 网络拓扑与地址分配表 …… 4 1.3 安装前准备事宜 …… 6 第二章 Nokia IP380 安装与配置 …… 7 2.1 概述 …… 7 2.2 初始化 nokia380 …… 7 2.3 设置 nokia 基本信息 …… 8 2.3.1 Nokia 端口 IP 地址设定 …… 8 2.3.2 设置网关路由 …… 8 2.3.3 设置 Nokia 平台时间 …… 9 2.3.4 设定 Nokia 高可用 VRRP 参数 …… 9 2.4 初始化 checkpoint …… 14 2.4.1 在 nokia 平台上 checkpoint 的安装与卸载 …… 14 2.4.2 初始化 checkpoint …… 16 第三章 管理服务器的安装与配置…… 17 3.1checkpoint smartcenter 的安装…… 18 3.1.1 安装前的准备 …… 18 3.1.2 安装步骤 …… 18 3.2 配置 checkpoint 对象和参数 …… 20 3.2.1 建立 sic …… 20 3.2.2 定义防火墙对象拓扑结构 …… 21 3.2.3 使用同样的步骤按照表 1 的参数建立 IP380B checkpoint gateway 对象。 …… 21 3.3 基于 nokia vrrp 或者 cluster 的设置…… 22 3.3.1 基于 Nokia VRRP 的设置 …… 22 3.3.2 为 nokia vrrp 定义策略 …… 22 3.3.3 高可用性的检查 …… 23 3.4nokia cluster 的设置 …… 23 3.5 暂时没有 …… 23 第四章 策略设定…… 24 4.1 概述 …… 24 4.2 netscreen 的策略 …… 24 4.3 经过整理后转换成 checkpoint 的策略 …… 24 4.4 设定策略 …… 24 4.4.1 定义主机对象 …… 24 4.4.2 定义网络对象 …… 25 4.4.3 定义组 …… 26 4.4.4 定义服务 …… 26 4.4.5 添加标准策略 …… 27 4.4.6 添加 NAT 策略…… 27 第五章 切换与测试…… 29 5.1 切换 …… 29 5.2 测试 …… 29 5.3 回退 …… 30 第六章 日常维护…… 31 6.1 防火墙的备份与恢复 …… 31 6.1.1 nokia 防火墙的备份与恢复方法 …… 31 6.1.2 checkpoint management 上的备份与恢复 …… 33 第一章 客户环境概述 1.1 概述 XXXXXX 公司因应企业内部的网络需求,对总部网络进行扩容改动,中心 防火墙从原来的 netscreen 换成两台 Nokia IP380,两台 nokia 互为热备。维持原 有的服务不变。 由于这次网络改动比较大,所以先离线进行安装和测试,最后再进行切换 1.2 网络拓扑与地址分配表 XXXXXX 改造前网络拓扑如下 改动后,XXX 将按照以下图进行实施 改动后, 给个设备及端口的地址分配入下表所示 IP 地址分配表(表 1) 管理服务器参数 IP 地址 防火墙各端口参数 端口 Eth1 用途 外网 口 Eth2 Eth3 Eth4 ? DMZ 内网 同步 口 gateway 静态路 由 172.16.100.5/30 172.16.100.6/30 172.16.100.1/30 10.101.1.101/24 10.101.1.102/24 10.101.1.1/24 192.168.11.1/24 192.168.11.2/24 Nokia380A Nokia380B 虚拟地址 1.3 安装前准备事宜 1.管理服务器硬件平台 CPU 奔腾 3 500 以上 内存 128 以上 硬盘 60M 以上 操作系统,windows 2000 server +SP4 补丁 2.网络连线 3.Checkpoint 及 nokia 管理软件 Checkpoint NG AI R55 安装包 for windows hotfix09 或以上 4.Nokia IP380 设备两台 内置 IPSO3.8 build 39 内置 checkpoint NG AI R55 安装包 第二章 Nokia IP380 安装与配置 2.1 概述 首先我们可以对两台 Nokia IP380 进行安装与配置,由于 Nokia 防火墙将会 替代 Netscreen,所以 Nokia 防火墙可以进行离线配置。配置步骤如下。 2.2 初始化 nokia380 1. 使用 nokia console 线,连接 nokia console 口和管理 pc 的串行端口,
燃气轮机性能指标主要影响因素及提高性能途径研究
燃气轮机性能指标主要影响因素及提高性能途径研究 摘要: 本文以9e燃机为例,概括介绍了国内已经投产的燃气轮机的主要性能指标,并通过对不同设计和运行条件下技术性能指标的对比,分析对燃气轮机性能指标产生影响的主要影响因素,从而总结和简述了提高性能指标的主要途径。 关键词: 燃气轮机;性能指标;功率;热耗率;影响因素;abstract:illustrated by 9e gas turbine, the main technical performance parameters of gas turbine in china are described, and with the comparison of the technical parameters under different design and operation condition, an analysis on the main influencing factors is presented, so as to summarizethe major way to improve the performance parameters. keywords: gas turbine; performance parameter; power; heat rate; influencing factor 中图分类号:th138.23 文献标识码:a文章编号:2095-2104(2012) 1.引言 燃气轮机是从本世纪50年代开始逐渐登上发电工业舞台的。但是由于当时机组的单机容量较小,而热效率又比较低,因而在电力系统中只能作为紧急备用电源和调峰机组使用。 60年代时欧美的大电网曾发生过电网瞬时解列的大事故,这些事
win7防火墙设置指南、多重作用防火墙策略以及设置方法
win7防火墙设置指南、多重作用防火墙策略以及设置方法 214小游戏https://www.sodocs.net/doc/0416957312.html,/ windows XP集成防火强常被视为鸡肋,不过随着vista和WIN7的发布,微软对于防火墙的两次升级让windows的firewall不再是系统的累赘,特别是win7的firewall,强悍的功能让微软的防火墙也有了“专业”的味道。 本文就教大家来了解一下windows7下的 firewall设置以及向你展示怎样对多重作用防火墙策略下对Firewall进行配置。Windows Firewall的演变过程。 一、windows防火墙的演变 Windows XP中的防火墙是一款简单、初级仅保护流入信息,拦截任何不是由你主动发启入站连接的软件--它是默认关闭的。SP2后它才被默认启动并可以通过组策略的方式由管理员进行配置。而 Vista Firewall 是建立在一个新的Windows Filtering Platform (WFP、Windows过滤平台)上、并通过Advanced Security MMC嵌入式管理单元添加了新的过滤外发信息的能力。在Windows 7中MS对firewall做了进一步的微调,使其更具可用性,尤其针对移动计算机,更是舔加了对多重作用防火墙策略的支持。 二、windows 7 firewall(防火墙)设置方法 与Vista相同的是,可以通过访问控制面板程序对Windows 7 firewall进行基础配置。与Vista不同的是,你还可以通过访问控制面板的方式对其进行高级配置(包括对出站连接过滤器的配置),而不是一定要创建空白MMC并加入嵌入式管理单元来实现。只是点击一下左侧面板里的高级配置选项。 更多的网络配置 Vista firewall允许你去选择是在公共网格上还是在专用网络中,而在Windows 7中你有三个选择--公用网络、家庭网络、办公网络。后两个选项是专用网络的细化。 如果你选择了“家庭网络”选项,你将可以建立一个“家庭组”。在这种环境中,“网路发现”会自动启动,你将可以看到网络中其它的计算机和设备,同时他们也将可以看到你的计算机。隶属于“家庭组”的计算机能够共享图片、音乐、视频、文档库以及如打印机这样的硬件设备。如果有你不想共享的文件夹在文档库中,你还可以排除它们。 如果你选择的是“工作网络”,“网路发现”同样会自动启动,但是你将不能创建或是加入“家庭组”。如果你的计算机加入了Windows域(通过控制面板--系统和安全--系统--高级系统配置--计算机名选项卡)并通过DC验证,那么防火墙将自动识别网络类型为域环境网络。 而“公用网络”类型是当你在机场、宾馆、咖啡馆或使用移动宽带网络联通公共wi-fi 网络时的适当选择,“网路发现”将默认关闭,这样其它网络中的计算机就不会发现你的共享而你也将不能创建或加入“家庭组”。 在全部的网络模式中,Windows 7 firewall都将在默认情况下拦截任何发送到不属于白名单中应用程序的连接。Windows 7允许你对不同网络类型分别配置。 多重作用防火墙策略 在Vista中,尽管你有公用网络和私用网络两个配置文件,但是只会有一个在指定的时间内起作用。所以如果你的计算机发生要同时连接两个不同网络的情况,那你就要倒霉啦。最严格的那条配置文件会被用户到所有的连接上,这意味着你可能无法在本地(私用)网络中做你想做的事,因为你是在公用网络在规则下操作。而在Windows 7 (和 Server 2008 R2)中,不同网络适配器上可以使用不同的配置文件。也就是说专用网络之间的网络连接受专用网络规则支配,而与公用网络之间的流量则应用公用网络规则。 起作用的是那些不显眼的小事 在很多事例中,更好的可用性往往取决于小的改变,MS听取了用户的意见并将一些“不
Check Point教程
Check point 防火墙基本操作手册 CheckPoint(中国) TEL:(86)10 8419 3348 FAX:(86)10 8419 3399 ?2010 Check Point Software Technologies Ltd. All rights reserved. Classification:
目录 目录 (2) 防火墙架构 (3) 防火墙的Web管理 (3) 配置IP: (4) 配置DNS和Host: (5) 配置路由: (5) 通过防火墙的管理客户端管理 (5) 添加防火墙 (7) 添加策略步骤 (10) IP节点添加 (10) 添加网段 (11) IPS的配置 (13) 更新IPS库 (14) 新建IPS动作库 (14) 应用控制 (16) 更新数据库 (16) 添加应用控制策略 (17) App Wike (18) 自定义添加应用 (18) QOS配置 (20) Qos策略的添加 (20) 日志工具的使用 (20) 筛选日志 (21) 临时拦截可以连接 (22) ?2010 Check Point Software Technologies Ltd. All rights reserved. Classification:
?2010 Check Point Software Technologies Ltd. All rights reserved. Classification: 防火墙架构 Check point 防火墙的管理是通过一个三层架构来实现的。首先我们可以在任意的机器上安装防火墙客户端控制台,然后利用控制台的图形化界面登录check point 的管理服务器,定义出各个网络对象,定义企业各条策略,最后下发到防火墙执行模块。具体实现过程见图示: 防火墙的Web 管理 首先打开Web 管理界面,出现登录界面:
CheckPoint 防火墙基本操作及应急措施
防火墙基本操作及应急措施陈世雄安全工程师 CCSE
议程 ?智能边界安全解决方案?CheckPoint 配置基础?常见问题及应急措施
Check Point 简介 ?最受信赖、最可依靠的互联网安全厂商 –我们致力发展安全领域——并且比任何厂商更优秀! –全球财富100企业中,100%企业使用我们的产品 –在防火墙和虚拟专用网络(VPN)市场中占有领导 地位 ?在全球 VPN/防火墙软件市场销售额中占70%份额 (Infonetics提供数据) ?VPN/防火墙软件市场占有率超过 54% (IDC提供数据) ?安全硬件设备市场份额中有 36% 为 Check Point 产品(由 Infonetics 提供) ?以客为本的企业原则 –业界领先的技术合作伙伴关系 –强大且广泛的渠道合作伙伴关系
状态检测 / FireWall-1 1993 OPSEC 1997 VPN-1 1998 Next Generation 2001 SmartDefense 2002 应用智能 2003 Check Point: 一直走在客户现实需求的前面 创新历程 1994 1995 1996 1999 2000 Web 智能 2004
我们的策略 2004上半年提供! ? 安全远程访问 ? Web 服务器保护 ? 统一认证 ? 一致性策略管理 ? 市场领先 ? 十年的成功史 ? 最新发布 - InterSpect 2.0 - Connectra 2.0 - Intergrity 6.0 ? Check Point InterSpect ? Zone Labs SMART 管理 无忧保护 边界 深入检查 智能 安全解决方案
防火墙的基本配置原则
本篇要为大家介绍一些实用的知识,那就是如何配置防火中的安全策略。但要注意的是,防火墙的具体配置方法也不是千篇一律的,不要说不同品牌,就是同一品牌的不同型号也不完全一样,所以在此也只能对一些通用防火墙配置方法作一基本介绍。同时,具体的防火墙策略配置会因具体的应用环境不同而有较大区别。首先介绍一些基本的配置原则。 一. 防火墙的基本配置原则 默认情况下,所有的防火墙都是按以下两种情况配置的: ?拒绝所有的流量,这需要在你的网络中特殊指定能够进入和出去的流量的一些类型。 ?允许所有的流量,这种情况需要你特殊指定要拒绝的流量的类型。可论证地,大多数防火墙默认都是拒绝所有的流量作为安全选项。一旦你安装防火墙后,你需要打开一些必要的端口来使防火墙内的用户在通过验证之后可以访问系统。换句话说,如果你想让你的员工们能够发送和接收Email,你必须在防火墙上设置相应的规则或开启允许POP3 和SMTP 的进程。 在防火墙的配置中,我们首先要遵循的原则就是安全实用,从这个角度考虑,在防火墙的配置过程中需坚持以下三个基本原则:(1). 简单实用:对防火墙环境设计来讲,首要的就是越简单越好。其实这也是任何事物的基本原则。越简单的实现方式,越容易理解和使用。而且是设计越简单,越不容易出错,防火墙的安全功能越容易得到保证,管理也越可靠和简便。 每种产品在开发前都会有其主要功能定位,比如防火墙产品的初衷就是实现网络之间的安全控制,入侵检测产品主要针对网络非法行为进行监控。但是随着技术的成熟和发展,这些产品在原来的主要功能之外或多或少地增加了一些增值功能,比如在防火墙上增加了查杀病毒、入侵检测等功能,在入侵检测上增加了病毒查杀功能。但是这些增值功能并不是所有应用环境都需要,在配置时我们也可针对具体应用环境进行配置,不必要对每一功能都详细配置,这样一则会大大增强配置难度,同时还可能因各方面配置不协调,引起新的安全漏洞,得不偿失。 (2). 全面深入:单一的防御措施是难以保障系统的安全的,只有采用全面的、多层次的深层防御战略体系才能实现系统的真正安全。在防火墙配置中,我们不要停留在几个表面的防火墙语句上,而应系统地看等整个网络的安全防护体系,尽量使各方面的配置相互加强,从深层次上防护整个系统。这方面可以体现在两个方面:一方面体现在防火墙系统的部署上,多层次的防火墙部署体系,即采用集互联网边界防火墙、部门边界防火墙和主机防火墙于一体的层次防御;另一方面将入侵检测、网络加密、病毒查杀等多种安全措施结合在一起的多层安全体系。 3). 内外兼顾:防火墙的一个特点是防外不防内,其实在现实的网络环境
Checkpoint防火墙测试用例
Checkpoint R65 Test Plan Revision 0.1 2009-10-14 Author: Peng Gong
Revision History
1‘FW MONITOR’ USAGE (7) 2TEST SUITES (7) 2.1R65I NSTALL &U NINSTALL (7) 2.1.1Create a v3 vap-group with vap-count/max-load-count set >1, Confirm R65 and related HFA could be installed on all vap within vap-group successfully (7) 2.1.2Create a v4 vap-group, confirm XOS would prompt correctly message and failure install would cause unexpected issue to system (9) 2.1.3Create a v5 vap-group, confirm XOS would prompt correctly message and failure install would cause unexpected issue to system (10) 2.1.4Create a v5_64 vap-group, confirm XOS would prompt correctly message and failure install would cause unexpected issue to system (11) 2.1.5Try to install R65 on a v3 vap-group and abort the install process, confirm nothing is left afterwards12 2.1.6Reduce max-load-count down to 1, confirm only one Vap is allowed to run R65 accordingly (13) 2.1.7Increase vap-count and max-load-count to maximum vap count and executing "application-update", confirm all the Vap are running R65 properly (14) 2.1.8Reduce vap-count to 3, Confirm the surplus Vap is stopped from running R65 and related partitions are removed from CPM (15) 2.1.9Enable/Disable IPv6 in vap-group and w/o ipv6-forwarding, confirm R65 wouldn't be affected. (16) 2.1.10Confirm the installed R65 could be stopped through CLI (17) 2.1.11Confirm the installed R65 could be started through CLI (18) 2.1.12Confirm the installed R65 could be restarted through CLI (18) 2.1.13Confirm the installed R65 could be reconfigured through CLI (19) 2.1.14Confirm checkpoint could be upgraded to R70 by CLI: application-upgrade (19) 2.1.15Confirm R65 could run properly while reload vap-group totally (20) 2.1.16Confirm R65 could run properly while reload all chassis totally (20) 2.1.17Create 2 vap groups, install R65 on both. Confirm R65 are running on both vap-groups without any mutual impact. (21) 2.1.18Confirm configure operation couldn't be allowed if some Vap don't run R65 properly. (23) 2.1.19Confirm uninstall/Configure operation couldn't be allowed if some Vap don't run properly. (24) 2.1.20Confirm start/stop/restart operation could works prope rly if R65 doesn’t run properly on some Vaps.25 2.1.21Confirm configure operation couldn't be allowed if vap-count>max-load-count causing some Vap don't run properly (26) 2.1.22Confirm uninstall operation couldn't be allowed if vap-count>max-load-count causing some Vap don't run properly (27) 2.1.23Confirm start/stop/restart operation couldn't be allowed if vap-count>max-load-count causing some Vap don't run properly (28) 2.1.24Confirm R65 could be uninstalled from vap-group (29) 2.1.25Confirm R65 installation package could be remove from XOS by CLI: application-remove (30) 2.2C HECKPOINT F EATURES (31) 2.2.1Create GW and Set SIC, get topology from Smart Dashboard (31) 2.2.2Configure Policy and push policy (31) 2.2.3Confirm R65 could be started/stoped/restarted by cp-command (32) 2.2.4Confirm different kinds of rule actions could work - allow/drop/reject (33) 2.2.5Verify different kinds of tracking actions could work properly - log/alert/account (33) 2.2.6Verify static NAT and hide NAT work properly (34) 2.2.7Verify default license is installed automatically (34) 2.2.8Verify some usual fw commands output - fw ver -k/ fw ctl pstat/ fw tab/ fw stat/ cphaprob stat /fw fetch /fw unloadlocal / fwaccel stat (34) 2.2.9Verify SXL status if enable/disable it (36) 2.2.10Verify the log information in SmartViewer are displayed correctly. (37) 2.2.11Verify the GW and its member information in SmartViewer are displayed correctly (37) 2.2.12Define a VPN Community between two GW; Confirm traffic can pass (37) 2.2.13Create a VPN client tunnel, make sure client can login and traffic is encrypted as it should (38) 2.2.14Enabling/Disabling SXL during connections (39) 2.2.15Fail over the active member during connections (39) 2.3I NTERFACE T EST-T RAFFIC MIXED WITH IPV6 AND IPV4 (39) 2.3.1Verify traffic pass through the interface without SXL - non vlan<--->vlan (tcp+udp+icmp) (39)
第一讲燃气轮机基本原理及9E燃机性能型号参数
第一讲:燃气轮机基本原理及9E燃机性能型号参数授课内容: 第一章:绪论 1):燃气轮机发电装置的组成 2):燃气轮机发展史 3):我国燃气轮机工业慨况 4):GE公司燃气轮机产品系列及其编号 第二章:燃气轮机热力学基础知识 1):工质的状态参数 2):理想气体状态方程 3):功和热量 第三章:燃气轮机热力循环 1):燃气轮机热力循环的主要技术指标 2):燃气轮机理想简单循环 3):燃气—蒸汽联合循环 第四章:9E燃机性能型号参数 1):PG9171E型燃机型号简介 2):PG9171E型燃机性能参数简介
第一章绪论 第一节燃气轮机发电装置的组成 燃气轮机是近几十年迅速发展起来的热能动力机械。现广泛应用的是按开式循环工作的燃气轮机。它不断地由外界吸入空气,经过压气机压缩,在燃烧室中通过与燃料混合燃烧加热,产生具有较高压力的高温燃气,再进入透平膨胀作功,并把废气排入大气。输出的机械功可作为驱动动力之用。因此,由压气机、燃烧室、透平再加上控制系统及基本的辅助设备,就组成了燃气轮机装置。如果用以驱动发电机供应电力,就成了燃气轮机发电装置。 (幻灯)
第二节 燃气轮机发展史 燃气轮机是继汽轮机和内燃机问世以后,吸取了二者之长而设计出来的,它
是内燃的,避免了汽轮机需要庞大锅炉的缺点;又是回转式的,免去了内燃机中将往复式运动转换成旋转运动而带来的结构复杂,磨损件多,运转不平稳等缺点。但由于燃气轮机对空气动力学和高温材料的要求超过其他动力机械,因此,发展燃气轮机并使之实用化,人们为之奋斗了很长时间。如果从1791年英国人约翰·巴贝尔(John Baber)申请登记第一个燃气轮机设计专利算起,经过了半个世纪的奋斗,到1939年,一台用于电站发电的燃气轮机(400OkW)才由瑞士BBC公司制成,正式投运。同时Heinkel工厂的第一台涡轮喷气式发动机试飞成功,这标志着燃气轮机发展成熟而进入了实用阶段·在此以后,燃气轮机的发展是很迅速的。由于燃气轮机本身固有的优点和其技术经济性能的不断提高,它的应用很快地扩展到了国民经济的很多部门· 首先在石油工业中,由于油田的开发和建设,用电量急剧增加·建造大功率烧煤电站不具备条件(没有煤炭,交通不便,水源紧张,施工困难等),周期也不能满足要求·而燃气轮机电厂功率不受限制,建造速度抉,对现场条件要求不高,油田有充足的可供燃用的气体和液体燃料·不少油田还利用开发过程中一时难以利用的伴生气作燃气轮机燃料,价格便宜,发电成本低,增加了燃气轮机的竞争力,所以在油田地区,燃气轮机装置被广泛应用,除用于发电外,还在多种生产作业申用燃气轮机带动压缩机(例如天然气管道输送,天然气回注,气田采油等)和泵(例如原油管道输送和注水等)。 其他工业部门,如炼油厂、石油化工厂、化工厂、造纸厂等等;它们不仅需要机械动力,而且需要大量热(例如蒸汽)。这时用燃气轮机来功热联供,在满足这两方面需要的同时,还能有效地节能,故应用发展较快。 实践证明,燃气轮机作为舰船推进动力,其优点显著,特别是排水量为数千
防火墙基础知识
防火墙基础知识 3.3 包过滤包过滤技术(Ip Filtering or packet filtering) 的原理在于监视并过滤网络上流入流出的Ip包,拒绝发送可疑的包。由于Internet 与Intranet 的连接多数都要使用路由器,所以Router成为内外通信的必经端口,Router的厂商在Router上加入IP Filtering 功能,这样的Router也就成为Screening Router 或称为Circuit-level gateway. 网络专家Steven.M.Bellovin认为这种Firewall 应该是足够安全的,但前提是配置合理。然而一个包过滤规则是否完全严密及必要是很难判定的,因而在安全要求较高的场合,通常还配合使用其它的技术来加强安全性。Router 逐一审查每份数据包以判定它是否与其它包过滤规则相匹配。(注:只检查包头的内容,不理会包内的正文信息内容) 过滤规则以用于IP顺行处理的包头信息为基础。包头信息包括: IP 源地址、IP目的地址、封装协议(TCP、UDP、或IP Tunnel)、TCP/UDP源端口、ICMP包类型、包输入接口和包输出接口。如果找到一个匹配,且规则允许这包,这一包则根据路由表中的信息前行。如果找到一个匹配,且规则允许拒绝此包,这一包则被舍弃。如果无匹配规则,一个用户配置的缺省参数将决定此包是前行还是被舍弃。*从属服务的过滤包过滤规则允许Router取舍以一个特殊服务为基
础的信息流,因为大多数服务检测器驻留于众所周知的TCP/UDP端口。例如,Telnet Service 为TCP port 23端口等待远程连接,而SMTP Service为TCP Port 25端口等待输入连接。如要封锁输入Telnet 、SMTP的连接,则Router 舍弃端口值为23,25的所有的数据包。典型的过滤规则有以下几种: .允许特定名单内的内部主机进行Telnet输入对话 .只允许特定名单内的内部主机进行FTP输入对话 .只允许所有Telnet 输出对话 .只允许所有FTP 输出对话 .拒绝来自一些特定外部网络的所有输入信息* 独立于服务的过滤 有些类型的攻击很难用基本包头信息加以鉴别,因为这些独立于服务。一些Router可以用来防止这类攻击,但过滤规则需要增加一些信息,而这些信息只有通过以下方式才能获
CheckPoint防火墙安装手册
防火墙安装操作手册By Shixiong Chen
一、准备安装介质和服务器 安装CheckPoint防火墙基于开放的服务器平台需要准备两点: 如果选用的是Checkpoint的硬件,UTM-1则不需要考虑这些问题。 第一,准备好服务器,服务器最好选择IBM\HP\或者其他大品牌厂商的硬件平台,并且事先确认该品牌和型号的服务器与CheckPoint软件兼容性,将网卡扩展至与真实火墙一致,服务器需要自带光驱。 第二,准备好CheckPoint防火墙安装介质,注意软件的版本号与真实环境火墙一致,同时准备好最新的防火墙补丁。 二、SecurePlatform系统安装过程 硬件服务器初始状态是裸机,将服务器加电后,设置BIOS从光盘启动,将CheckPoint软件介质放入光驱,然后出现如下界面: 敲回车键盘开始安装。出现如下界面,选择OK,跳过硬件检测。
选择安装的操作系统类型,根据自己防火墙操作系统的版本选择。SecurePlatform Pro是带有高级路由和支持Radius的系统版本。 选择键盘支持的语言,默认选择US,按TAB键,继续安装。 配置网络接口,初始我们配置外网接口即可,选择eth0配置IP, 输入IP地址、子网掩码、以及默认网关,然后选择OK,继续。 选择OK.开始格式化磁盘。
格式化完成后开始拷贝文件,最后提示安装完成,按照提示点击OK.系统会自动重新启动。然后出现登陆界面,如下所示。 第一次登陆系统,默认账号和密码都是admin,登陆后需要出入新的密码和管理员账号。 三、CheckPoint防火墙软件安装过程
运行sysconfig 命令,启动安装防火墙包。选择n,继续安装。 打开网络配置页面,选择1,配置主机名,选择3配置DNS服务器,选择4配置网络,选择5配置路由,注意要与生产线设备的配置保持一致,特别是路由要填写完整,主机名、接口IP和子网掩码要填写正确。 配置完成后,选择n,下一步继续配置,如下图所示,选择1配置时区(选择5,9,1),选择2配置日期,选择3配置本地系统时间,选择4查看配置情况。注意配置时间和日期的格式。完成后选择n, 然后会出现提示是否从tftp服务器下载安装软件,选择n.然后出现如下界面,选择N,继续安装。 选择Y,接受安装许可协议。
防火墙基础知识
(一) 防火墙概念 防火墙不只是一种路由器、主系统或一批向网络提供安全性的系统。相反,防火墙是 一种获取安全性的方法;它有助于实施一个比较广泛的安全性政策,用以确定允许提供的服务和访问。就网络配置、一个或多个主系统和路由器以及其他安全性措施(如代替 静态口令的先进验证)来说,防火墙是该政策的具体实施。防火墙系统的主要用途就是 控制对受保护的网络(即网点)的往返访问。它实施网络访问政策的方法就是逼使各连接 点通过能得到检查和评估的防火墙。 ____________ ___________ | | | | | Computer | | Computer | |__________| |___________| ____________ ________ | | |应| |Packet | ______|________________|__________| 用|_____|Filter |___Internet | 网| |Router | 网点系统| 关| |________| |__________| 路由器和应用网关防火墙范例 防火墙系统可以是路由器,也可以是个人主机、主系统和一批主系统,专门把网 络或子网同那些可能被子网外的主系统滥用的协议和服务隔绝。防火墙系统通常位于等级较高的网关如网点与Internet的连接处,但是防火墙系统可以位于等级较低的网关, 以便为某些数量较少的主系统或子网提供保护。 防火墙基本上是一个独立的进程或一组紧密结合的进程,运行于Router or Server 来控制经过防火墙的网络应用程序的通信流量。一般来说,防火墙置于公共网络(如Inter- -net)人口处。它可以看作是交通警察。它的作用是确保一个单位内的网络与Internet之间所有的通信均符合该单位的安全方针。这些系统基本上是基于TCP/IP,并与实现方法有关,它能实施安全路障并为管理人员提供对下列问题的答案: * 谁在使用网络? * 他们在网上做什么? * 他们什么时间使用过网络?
临界状态滑坡土层参数反演在工程中的应用
文章编号:1009-6825(2013)01-0048-02 临界状态滑坡土层参数反演在工程中的应用 收稿日期:2012-10-26作者简介:王树州(1983-),男,硕士,工程师; 刘强(1978-),男,工程师 王树州 刘强 (安徽省交通规划设计研究院有限公司,安徽合肥230008) 摘 要:针对芜湖至铜陵高速K51+354 K51+500段出现的裂缝及下挫现象,分析了其产生变形的原因,通过不平衡推力法算出 滑坡剩余下滑力, 提出了采用抗滑桩结合挡土墙支护边坡的方案,并在工程运用中得到了很好的效果。关键词:滑坡,临界状态,反演,裂缝及下挫,不平衡推力法 中图分类号:TU435 文献标识码:A 0引言 随着国民经济的飞速发展,大量铁路、公路、矿山等设施的修建,特别是丘陵和山区建设中,人类工程活动中开挖和堆填的边坡数量会越来越多, 高度也将越来越大。如北京—福州高速公路福建段200余千米内高度大于30m 的边坡多达150多处。由于地质条件复杂, 加之人类改造自然规模愈来愈大,设计施工方法不当,高边坡开挖后发生变形和造成灾害的事故频繁发生,给工程运营和人身安全带来很大隐患。 芜湖至铜陵高速K51+354 K51+500为开挖路段,右侧挖方较长,坡高较大,最大坡高31m 。该项目已建成运营近三年时间,于2010年4月K51+420 K51+480段右侧一级坡出现裂缝宽2cm 5cm ,一级坡护面墙局部开裂,二级坡裂缝宽10cm 30cm ,二级坡平台下挫20cm 40cm ,估计松动方量4000m 3,坡 脚未出现剪出口。该滑坡体处于蠕动变形阶地, 若遇到暴雨天气,雨水下渗,有可能会加速下滑,危及人的生命安全。 1滑坡区工程地质概况1.1地形地貌 边坡区地貌属低山丘陵区,区内地形较简单,岗凹相间内,岗丘顶部浑圆,坡面平缓,覆盖层主要为残坡积层,凹地上部覆盖第四系全新统冲积层。 1.2地层结构及岩土体特征 滑坡区上部覆盖层为第四系中更新统残坡积层(Q el +dl 2 )的粗粒土和高液限粘土,粗砾土层厚7.5m 10.7m ,高液限粘土层厚8.0m 12.4m ,工程性质差;下伏基岩为三叠系下统南陵湖组(T 1n )灰岩。 1.3水文地质特征 滑坡区主要赋存少量残坡积松散层孔隙水,主要来源于大气 降水补给,季节性变化较大,但由于上部的碎石土夹砂砾石及少量细粒土,渗透性较好,降雨时大量的地表水下渗,而中部高液限 粘土及下部基岩为相对不透水层,致使高液限粘土含水量增高, 而高液限粘土遇水后性质变差,形成软弱层,对边坡稳定不利,滑坡区应设置好防渗及排水措施。 2滑坡基本特征及成因分析2.1 滑坡基本特征 滑坡区位于K51+354 K51+500的右侧,整体坡度为36?, 坡形整体呈上缓下陡,只有护面墙护坡,如图1所示。该滑坡分三级台阶,第三级台阶的护面墙已经损坏,可能是导致降雨入渗的主要原因。第一、第二级台阶的护面墙也有拉裂地方。滑坡区右侧一级坡出现裂缝宽2cm 5cm ,二级坡裂缝宽10cm 30cm ,二级坡平台下挫20cm 40cm ,如图2所示。 图1 滑坡区地貌特征 图2第二级台阶开裂下挫 2.2滑坡成因分析 1)雨水下渗。边坡排水沟、截水沟日渐淤积堵塞,护面墙开裂,导致降雨下渗不能及时的排出坡体,使得坡体含水率增高。而第一级、第二级台阶主要分布着高液限粘土,富含高岭土,具有膨胀性,当坡体含水率增高时,坡体内土体膨胀,膨胀力使得护面墙开裂,同时土体的抗剪强度降低。三级坡的粗粒土夹有少量的 砾石, 渗透性较好,又是雨水下渗的良好通道。2)支挡不足。该边坡坡度较高,1?1 1?1.3的坡率只能保证每一级台阶是安全的,整体边坡是欠稳定的。整个边坡缺乏有效的支挡, 仅仅修筑护面墙是不能抵抗坡体变形产生的下滑力櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅櫅 。On engineering features of collapsible loess ZHANG Ai-fang (Shanxi Jinzheng Construction Engineering Program Management ,Co.,Ltd ,Hejin 043304,China ) Abstract :According to the distribution regions ,the horizontal and vertical distribution features of the collapsible loess of Shanxi Aluminum Plant ,the paper illustrates the conditions for the self-weight collapsibility ,features for the moment of the self-weight collapsible deformation after being soaked in water ,as well as the scopes for the deformation ,identifies the deformation features of the natural and compacted foundation un-der the measurement of additional collapsible volume ,and concludes the measured self-weight collapsible volume is less than the one of the in-door test calculation ,and the adopted correction factors in the new regulation is fundamentally consistent.Key words :collapsibility deformation ,subsidiary stress ,self-weight collapsible volume · 84·第39卷第1期2013年1月 山西 建筑 SHANXI ARCHITECTURE Vol.39No.1Jan.2013
相关文档
- CheckPoint防火墙配置
- CheckPoint防火墙安装手册
- Checkpoint防火墙安全配置手册V1.1
- CheckPoint防火墙配置
- CheckPoint防火墙故障处理流程
- CheckPoint防火墙安全配置基线
- CheckPoint 防火墙 双机 HA 实施 方案
- 11.CheckPoint 防火墙管理中心高可用性操作手册
- Check Point 防火墙优势
- Check point 防火墙基本操作手册
- Checkpoint防火墙测试用例
- 如何恢复Checkpoint UTM-1 270防火墙到出厂设置
- CheckPoint防火墙配置
- CheckPoint防火墙配置
- checkpoint防火墙组建ClusterXL+CoreXL+SecureXL
- checkpoint防火墙培训
- CheckPoint防火墙配置
- Checkpoint防火墙安全配置指南
- Checkpoint防火墙安全配置手册V11
- CheckPoint防火墙配置
