Amperometric sensor based on NASICON and NO oxidation

Solid State Ionics 136–137(2000)583–588
https://www.sodocs.net/doc/db18798881.html,/locate/ssi
Amperometric sensor based on NASICON and NO oxidation
catalysts for detection of total NO x in atmospheric environment
a a
b ,a *Masaki Ono ,Kengo Shimanoe ,Norio Miura ,Noboru Yamazoe
a Department of Molecular and Material Sciences ,Graduate School of Engineering Sciences ,Kyushu University ,Kasuga -shi ,
Fukuoka 816-8580,Japan
b Advanced Science and Technology Center for Cooperative Research ,Kyushu University ,Kasuga -shi ,Fukuoka 816-8580,Japan Abstract
A NASICON based amperometric sensor was designed for the detection of total NO x (NO 1NO )in air.To make the 2device sensitive to both NO and NO,the sensing electrode (Au)was covered with NO oxidation catalysts.With the sensing 2electrode potential ?xed at 2150mV vs.reference electrode (Au),the device attached with double catalyst layers of WO 3and Pt(0.5wt%)-loaded SiO produced current response proportional to the concentration of NO or NO up to 1ppm at 221508C.In addition,the proportionality constants (sensitivity)were almost the same to NO and NO .The current responses 2were barely affected by the coexistence of CO or H O.?2000Elsevier Science B.V .All rights reserved.
22Keywords :Total-NO x sensor;NaNO ;NASICON;NO oxidation catalyst;WO ;Pt–SiO ;Amperometric sensor;Environmental monitoring
2321.Introduction
more stable form at room temperature.The con-centration and composition of atmospheric NO x are Nitrogen oxides (NO x :NO and NO )are typical
variable,depending on the location,time and weath-2air-pollutants which cause acid rain and photochemi-
er conditions of the environmental site concerned.cal smog.Their concentrations in the atmospheric air
For example,NO usually surpasses NO in urban 2are currently measured by using analytical instru-
areas where there are many NO x emission sources.ments based on chemical luminescence or the
Several potentiometric devices to detect NO or 2Saltzman method,but those instruments are rather
NO have been proposed:the conventional concen-expensive and large.Thus,inexpensive compact
tration type [1–3]or unconventional types using a sensors are highly needed for monitoring environ-
metal salt auxiliary phase [4–6]or metal oxide mental NO x .NO x is emitted into environments
electrodes [7–11].Noticeably,the device using an mostly as NO from combustion furnaces and au-
NaNO -based auxiliary phase could detect as dilute 2tomobile engines,and after emission,the NO is
as 5ppb NO in air [6].Various amperometric 2gradually converted to NO ,a thermodynamically
devices have also been proposed [12–24],aimed at 2obtaining more precise concentration data for NO or 2NO.However,it was not possible to detect sub-ppm *Corresponding author.Tel.:181-92-583-7538;fax:181-92-
NO x in air amperometrically until we recently 575-2318.
1E -mail address :norioigz@mbox.nc.kyushu-u.ac.jp (N.Miura).reported a device using NASICON (Na -super-0167-2738/00/$–see front matter ?2000Elsevier Science B.V .All rights reserved.
PII:S0167-2738(00)00341-6
584M.Ono et al./Solid State Ionics136–137(2000)583–588
ionic-conductor)[25,26].The device could detect each Au electrode was ca.10m m.The Au counter
sub-ppm NO but was insensitive to NO.These electrode was covered with a layer of NaNO(ca.
22 features are well suited for NO sensing but need to200m m thick).This assembly was exactly the same
2
be altered for NO or total NO x sensing.To meet the as the amperometric NO sensor previously reported
2
latter mission,in this paper the device was attached[25,26].To obtain the device attached with a single
with catalysts to convert NO to NO over its sensing catalyst layer,WO was deposited on the sensing
23
electrode.The resulting device worked well as an electrode as follows.An aqueous paste of
amperometric sensor for detecting total NO x,as(NH)W O?5H O was applied on the sensing
41012412
described below.electrode and heated at6008C for5h to form a WO
3
layer(ca.50m m thick).In order to fabricate the
device attached with double catalyst layers,the WO
3 2.Experimental layer was stacked with a second catalyst layer(ca.
300m m thick)of Pt(0.5wt%)-loaded SiO:the
2
2.1.Fabrication of sensor devices second catalyst powder was mixed with a-terpineol
and ethyl cellulose,and the resulting paste was
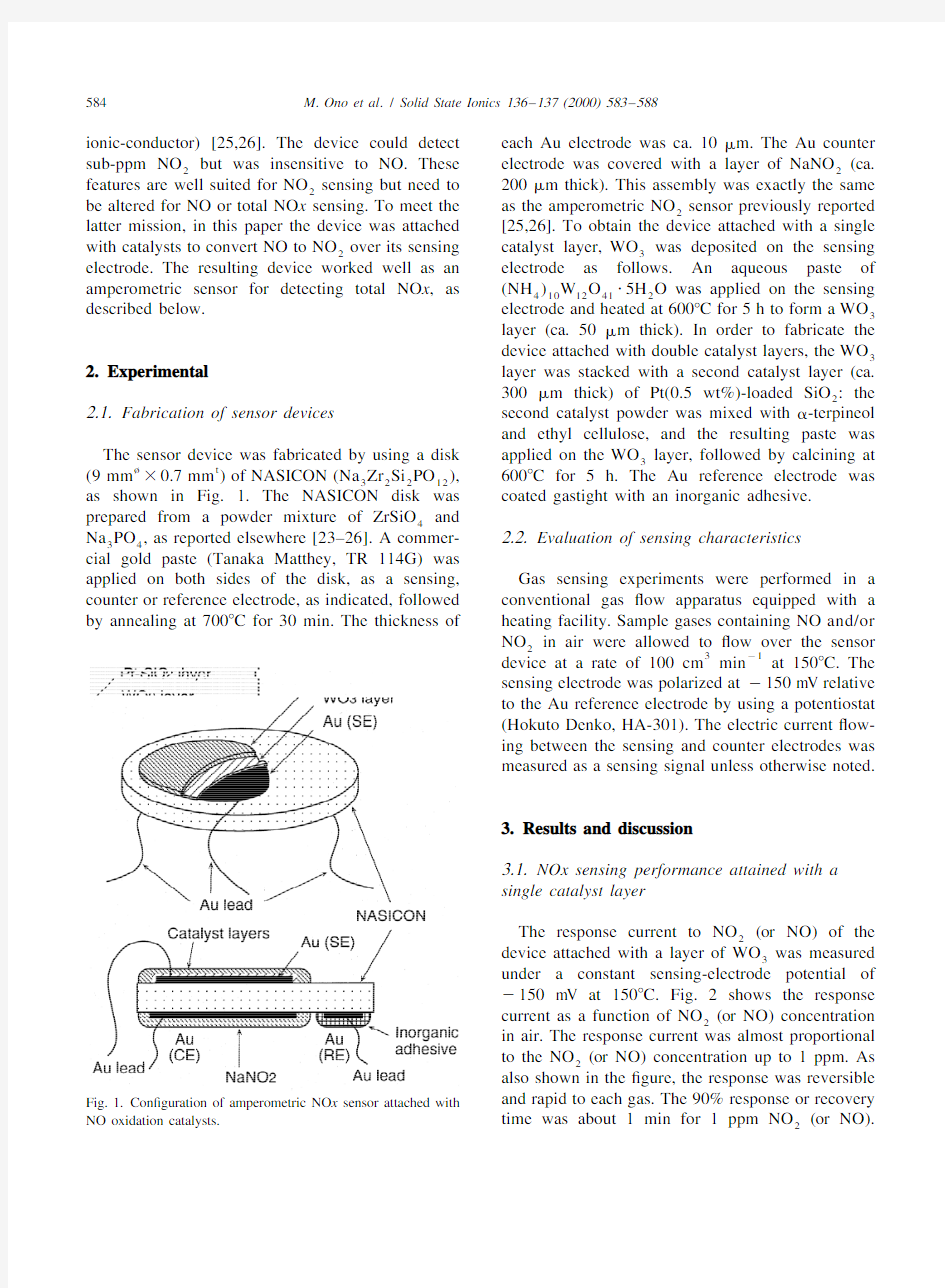
The sensor device was fabricated by using a disk applied on the WO layer,followed by calcining at
3
?t
(9mm30.7mm)of NASICON(Na Zr Si PO),6008C for5h.The Au reference electrode was
32212
as shown in Fig. 1.The NASICON disk was coated gastight with an inorganic adhesive.
prepared from a powder mixture of ZrSiO and
4
Na PO,as reported elsewhere[23–26].A commer- 2.2.Evaluation of sensing characteristics 34
cial gold paste(Tanaka Matthey,TR114G)was
applied on both sides of the disk,as a sensing,Gas sensing experiments were performed in a counter or reference electrode,as indicated,followed conventional gas?ow apparatus equipped with a by annealing at7008C for30min.The thickness of heating facility.Sample gases containing NO and/or
NO in air were allowed to?ow over the sensor
2
321
device at a rate of100cm min at1508C.The
sensing electrode was polarized at2150mV relative
to the Au reference electrode by using a potentiostat
(Hokuto Denko,HA-301).The electric current?ow-
ing between the sensing and counter electrodes was
measured as a sensing signal unless otherwise noted.
3.Results and discussion
3.1.NOx sensing performance attained with a
single catalyst layer
The response current to NO(or NO)of the
2
device attached with a layer of WO was measured
3
under a constant sensing-electrode potential of
2150mV at1508C.Fig.2shows the response
current as a function of NO(or NO)concentration
2
in air.The response current was almost proportional
to the NO(or NO)concentration up to1ppm.As
2
also shown in the?gure,the response was reversible
and rapid to each gas.The90%response or recovery Fig.1.Con?guration of amperometric NO x sensor attached with
NO oxidation catalysts.time was about1min for1ppm NO(or NO).
2
M .Ono et al ./Solid State Ionics 136–137(2000)583–588585
Fig. 2.Dependence of response current to NO or NO con-
2Fig.3.Polarization curves of the device attached with a double centration in air for the device attached with a single layer of WO 3
layer of WO and 0.5wt%Pt-loaded SiO in air and in the sample 32(2150mV ,1508C).
gases containing various concentrations of NO or NO at 1508C.2
However,the sensitivity (slope)to NO was smaller
than that to NO .This indicates that the catalytic
2conversion of NO to NO was not completed over
2the WO layer.
33.2.Sensing characteristics attained with double
catalyst layers
In order to improve the NO conversion ef?ciency,
a layer of 0.5wt%Pt-loaded SiO (Pt–SiO )was
22stacked additionally on the layer of WO .For the
3resulting device with double catalyst layers,polariza-
tion curves were measured under the presence of 0,
0.5and 1ppm NO (or NO)in air at 1508C.As
2shown in Fig.3,the current under air remains almost
zero over the sensing-electrode potential range from
Fig. 4.Dependence of response current to NO or NO con-2centration in air for the device attached with a double layer of ca.0to 2300mV ,whereas it shifts upward under
WO and 0.5wt%Pt-loaded SiO (2150mV ,1508C).32the presence of NO (or NO)over the same potential
2range.The increment of current is seen to be almost
proportional to the concentration of NO (or NO).It
response or recovery,as also indicated in Fig. 4.2is also noted that the polarization curves are almost
These results assure that the double catalyst layers coincide at the same concentrations of NO and NO.
function well for converting NO to NO .22The current measured at 2150mV was proportional
Fig.5compares the response current to NO and 2to the concentration of NO or NO up to 1ppm,as
NO (0.1ppm each)among the four devices,un-2shown in Fig. 4.In addition,the proportionality
attached or attached with WO and/or Pt–SiO 32constants (sensitivity)were almost the same for both
catalysts.The catalyst-free device was sensitive to NO and NO .The response transients to NO and
NO only,as previously reported [25,26].The device
222NO were fairly sharp,taking about 1min for 90%attached with WO gave a larger response current to
3
588M.Ono et al./Solid State Ionics136–137(2000)583–588
[6]N.Miura,S.Yao,Y.Shimizu,N.Yamazoe,Solid State Ionics 4.Conclusions
70/71(1994)572.
[7]Y.Shimizu,K.Maeda,Chem.Lett.1996(1996)117.
An amperometric total NO x sensor was obtained
[8]N.Miura,G.Lu,N.Yamazoe,H.Kurosawa,J.Hasei,J. by applying double layers of NO oxidation catalyst,Electrochem.Soc.L33(1996)143.
WO and Pt(0.5wt%)-loaded SiO,over the sensing[9]N.Miura,H.Kurosawa,M.Hasei,G.Lu,N.Yamazoe,Solid 32
State Ionics86/88(1996)1069.
Au electrode.The response current was linear to the
[10]G.Lu,N.Miura,N.Yamazoe,J.Mater.Chem.7(1997) concentration of NO and NO and was almost the
21445.
same to both,in the concentration range from0to1
[11]G.Lu,N.Miura,N.Yamazoe,Ionics4(1998)16.
ppm at1508C at a sensing electrode potential of[12]K.Nagashima,T.Hobo,Solid State Ionics40/41(1990)
2150mV vs.the Au reference electrode.The NO480.
2
[13]M.J.Tierney,H.L.Kim,Anal.Chem.65(1993)3435. sensing mechanism seems to involve electrochemical
[14]M.J.Tierney,H.L.Kim,M.Madou,T.Otagawa,Sensors formation and decomposition of NaNO at the
2and Actuators B13/14(1993)408.
sensing and counter electrode,respectively.The
[15]K.Y.Ho,M.Miyayama,H.Yanagida,J.Ceram.Soc.Japan porous layers of catalysts and electrode may work as104(1996)995.
a gas diffusion barrier.Although the cross-sen-[16]S.Somov,G.Reinhardt,U.Guth,W.Gopel,Sensors and
¨
Actuators B35/36(1996)409.
sitivities to S-containing species and halides for the
[17]J.S.Do,R.Y.Shieh,Sensors and Actuators B37(1996)19. sensor has not been examined so far,the device may
[18]G.Alberti, F.Cherubini,R.Palombari,Sensors and Ac-meet actual environmental monitoring applications,
tuators B37(1996)131.
with minor cross-sensitivity to CO,water vapor and[19]https://www.sodocs.net/doc/db18798881.html,ngmaier,F.Opekar,Z.Samec,Sensors and Actuators B
2
oxygen.41(1997)1.
[20]G.Lu,N.Miura,N.Yamazoe,Sensors and Actuators B52
(1998)169.
[21]G.Lu,N.Miura,N.Yamazoe,J.Appl.Electrochem.28 References
(1998)1009.
[22]N.Miura,G.Lu,M.Ono,N.Yamazoe,Solid State Ionics [1]M.Gauthier, A.Chamberland,J.Electrochem.Soc.124117(1999)283.
(1977)1579.[23]N.Miura,M.Iio,G.Lu,N.Yamazoe,J.Electrochem.Soc.
¨
[2]G.Hotzel,W.Weppner,Solid State Ionics18/19(1986)143(1996)L241.
1223.[24]N.Miura,M.Iio,G.Lu,N.Yamazoe,Sensors and Actuators [3]M.R.M.Jiang,M.T.Weller,Sensors and Actuators B30B35/36(1996)124.
(1996)3.[25]N.Miura,M.Ono,K.Shimanoe,N.Yamazoe,J.Appl.
[4]N.Rao,C.M.van den Bleek,J.Schoonman,Solid State Electrochem.28(1998)863.
Ionics52(1992)339.[26]N.Miura,M.Ono,K.Shimanoe,N.Yamazoe,Sensors and [5]N.Miura,S.Yao,Y.Shimizu,N.Yamazoe,Sensors and Actuators B49(1998)101.
Actuators B13/14(1993)387.
相关文档
- 最新小学数学课程标准(完整解读)
- 小学数学新课程标准(修改稿——)解读
- 最新(新版)小学数学新课程标准解读
- 修订版小学数学课标解读
- 数学新课标解读
- 高中数学新课程标准(解读)
- (完整版)人教版四年级数学新课标解读
- 《小学数学新课标解读》学习心得体会(3篇)
- 小学数学新课标解读学习心得体会
- 小学数学新课程标准解读
- 最新小学数学课程标准(完整解读)
- 《义务教育数学课程标准》 版 解读
- 最新初中数学课程标准解读(课堂PPT)
- 最新小学数学课程标准(完整解读)
- 新课程标准数学学科解读
- 2011版小学数学课程标准解读(全)
- (新版)小学数学新课程标准解读
- 小学数学新课程标准解读ppt课件
- (完整版)人教版小学数学新课程标准
- 小学数学新课标解读
