Edge flames and partially premixed combustion in diffusion flame quenching

Edge Flames and Partially Premixed Combustion in
Diffusion Flame Quenching
VALE′RIE FAVIER and LUC VERVISCH*
Institut National des Sciences Applique′es de Rouen,UMR-CNRS-6614/CORIA,Campus du Madrillet—Avenue de l’Universite′—BP8,76801Saint Etienne du Rouvray Cedex,France
The aim is to focus on the development of partially premixed combustion after diffusion?ame quenching.To this end,the quenching of a planar two-dimensional diffusion?ame is studied by using numerical simulation. A?ame hole is obtained by submitting the reaction zone to a high strain and scalar dissipation rate resulting from the interaction between vorticity and upstream triple?ame which stabilizes the diffusion?ame.The set of control parameters is chosen so that effects of unsteadiness are not expected during quenching,thus, extinction appears for a scalar dissipation rate that is well predicted by one-dimensional?amelet theory. Backward propagating edge?ames develop at both extremities of the quenched zone,whereas the combustion regime evolves from diffusion to partially premixed.From the results and the transport equation for a partially premixed fraction,a cross-scalar dissipation rate is introduced as a direct measure of the extent of partial premixing in non-premixed systems.For unity Lewis number,it is shown that the maximum burning rate measured in a one-dimensional planar stoichiometric premixed?ame may be used as a reference for a diffusion ?ame close to extinction and also later when edge?ames and triple?ames are formed.Finally,the simulations suggest that the scalar dissipation rate controlling the growth of the?ame hole is lower than the one that should be applied to?rst quench the?ame.?2001by The Combustion Institute
NOMENCLATURE
c Progress variable
D Diffusion coef?cient
D a Damko¨hler number
?Cross-scalar dissipation rate
l v Length of the vortices
n i Normal vector to the iso-Y i
R Radius of the vortices
S TF o Triple?ame burning velocity
t Time
u Streamwise component of the velocity v?Velocity of the vortices
(x,y)Coordinates
Y i Mass fraction
Z Mixture fraction
Greek Symbols
?Heat release parameter
?Zel’dovich number
?*q Quenching length
?Partially premixed fraction
?c Chemical time
??Diffusive time
?Angle between species gradients
?˙i Consumption rate of species i Superscripts
M Frozen?ow mixing
*1D reference?ame
?Average
Subscripts
burnt Burnt gases
fresh Fresh gases
Diff Trailing diffusion?ame
Ed Edge of reaction zone
o Feeding stream
q Quenching condition
s Stoichiometric mixture INTRODUCTION
Finite rate chemistry effects and quenching of diffusion?ames have been the subject of many studies[1–14].These have been motivated by the need to understand intermediate combus-tion regimes,ranging from fast chemistry to fast micromixing producing extinction of the reac-tion zones.In non-premixed systems,local?ow conditions promoting quenching of diffusive-reactive layers prohibit stabilization or develop-ment of the?ame,partial extinction also modi?es pollutant formation[15].As a consequence,
*Corresponding author.E-mail:vervisch@coria.fr.
COMBUSTION AND FLAME125:788–803(2001) 0010-2180/01/$–see front matter?2001by The Combustion Institute
PII S0010-2180(00)00242-X Published by Elsevier Science Inc.
diffusion ?ame quenching controls many prop-erties of burners and furnaces and the develop-ment of new strategies for Reynolds Averaged Simulation (RANS)or Large Eddy Simulation (LES)of ?ames requires a precise knowledge of these quenching mechanisms [16–18].
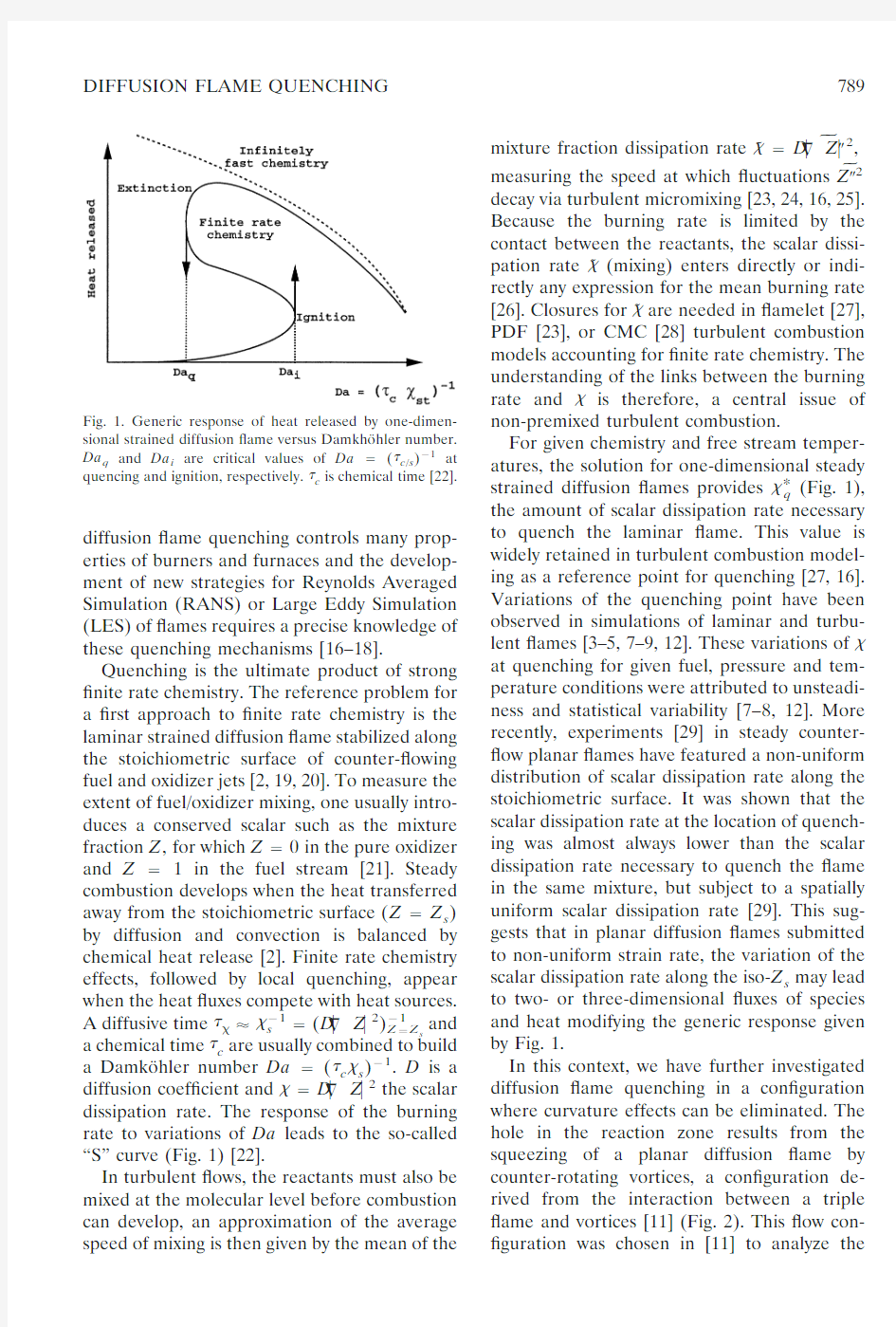
Quenching is the ultimate product of strong ?nite rate chemistry.The reference problem for a ?rst approach to ?nite rate chemistry is the laminar strained diffusion ?ame stabilized along the stoichiometric surface of counter-?owing fuel and oxidizer jets [2,19,20].To measure the extent of fuel/oxidizer mixing,one usually intro-duces a conserved scalar such as the mixture fraction Z ,for which Z ?0in the pure oxidizer and Z ?1in the fuel stream [21].Steady combustion develops when the heat transferred away from the stoichiometric surface (Z ?Z s )by diffusion and convection is balanced by chemical heat release [2].Finite rate chemistry effects,followed by local quenching,appear when the heat ?uxes compete with heat sources.
A diffusive time ????s ?1?(D ??Z ?2)Z ?Z s ?1
and a chemical time ?c are usually combined to build a Damko ¨hler number Da ?(?c ?s )?1.D is a diffusion coef?cient and ??D ??Z ?2the scalar dissipation rate.The response of the burning rate to variations of Da leads to the so-called “S”curve (Fig.1)[22].
In turbulent ?ows,the reactants must also be mixed at the molecular level before combustion can develop,an approximation of the average speed of mixing is then given by the mean of the
mixture fraction dissipation rate ???D ??Z ???2,measuring the speed at which ?uctuations Z ?2
?decay via turbulent micromixing [23,24,16,25].Because the burning rate is limited by the contact between the reactants,the scalar dissi-pation rate ??(mixing)enters directly or indi-rectly any expression for the mean burning rate [26].Closures for ??are needed in ?amelet [27],PDF [23],or CMC [28]turbulent combustion models accounting for ?nite rate chemistry.The understanding of the links between the burning rate and ?is therefore,a central issue of non-premixed turbulent combustion.
For given chemistry and free stream temper-atures,the solution for one-dimensional steady strained diffusion ?ames provides ?*q (Fig.1),the amount of scalar dissipation rate necessary to quench the laminar ?ame.This value is widely retained in turbulent combustion model-ing as a reference point for quenching [27,16].Variations of the quenching point have been observed in simulations of laminar and turbu-lent ?ames [3–5,7–9,12].These variations of ?at quenching for given fuel,pressure and tem-perature conditions were attributed to unsteadi-ness and statistical variability [7–8,12].More recently,experiments [29]in steady counter-?ow planar ?ames have featured a non-uniform distribution of scalar dissipation rate along the stoichiometric surface.It was shown that the scalar dissipation rate at the location of quench-ing was almost always lower than the scalar dissipation rate necessary to quench the ?ame in the same mixture,but subject to a spatially uniform scalar dissipation rate [29].This sug-gests that in planar diffusion ?ames submitted to non-uniform strain rate,the variation of the scalar dissipation rate along the iso-Z s may lead to two-or three-dimensional ?uxes of species and heat modifying the generic response given by Fig.1.
In this context,we have further investigated diffusion ?ame quenching in a con?guration where curvature effects can be eliminated.The hole in the reaction zone results from the squeezing of a planar diffusion ?ame by counter-rotating vortices,a con?guration de-rived from the interaction between a triple ?ame and vortices [11](Fig.2).This ?ow con-?guration was chosen in [11]to analyze
the
Fig.1.Generic response of heat released by one-dimen-sional strained diffusion ?ame versus Damkho ¨hler number.
Da q and Da i are critical values of Da ?(?c /s )?1at quencing and ignition,respectively.?c is chemical time [22].
789
DIFFUSION FLAME QUENCHING
dynamics of a leading edge in lifted ?ame stabilization [30,31].In such lifted ?ames,the ?ame base moves with the turbulence and some reaction zone extremities are convected,so that they avoid zones of intense micromixing featur-ing large values of ?(Fig.2,top).Nonetheless,when combustion is sustained by a ?ame base [30],other reaction zone extremities are trans-ported along a trajectory where they must be able to endure a strong increase in ?(Fig.2,top).Simulating the pinching of a triple ?ame was found to mimic this situation [11].For some conditions,the triple ?ame avoids the interac-tion and moves backwards along the stoichio-metric line.Other conditions lead to an up-stream propagation of combustion through the vortices,then the trailing diffusion ?ame is submitted to an unsteady distribution of Z featuring a local increase of ?,eventually fol-lowed by quenching.This synthetic and very simpli?ed con?guration allows planar diffusion ?ame quenching to be studied (Fig.2,bottom).In the simulations,the creation of a hole in a planar diffusion ?ame is associated with the birth of two reaction zone extremities similar to the so called “edge ?ame”discussed in [32].After primary quenching of the diffusion ?ame,these edge ?ames move backwards,increasing the length of the ?ame hole.During their backward propagation,where the edge ?ames escape from high strain,the scalar dissipation rate bordering the quenched location decreases and the edge ?ames,initially burning in a diffusive regime,progressively evolve into triple ?ames promoting partially premixed combus-tion.To quantify the local degree of fuel/air premixing,along with the transition from diffu-sion to partially premixed combustion,the bal-ance equation for a partially premixed fraction ?is introduced.Here ??Y F Y O is de?ned as the product of fuel and oxidizer local concen-trations:in?nitely fast chemistry in a diffusion ?ame would correspond to ??0.Straining a fast burning diffusion ?ame increases ?(Z s ),up to a quenching value ?q ,that is a measure of the leakage of the reactants through the stoichio-metric surface.The transport equation for ?introduces a cross-scalar dissipation rate ?,which is shown to be useful as a direct indicator of the existence of triple ?amelets.?measures the extent of partially premixed combustion in nonpremixed systems.
For unity Lewis number diffusion edge ?ames and the partially premixed triple ?ames,
which
Fig.2.Sketch of simulations of two-dimensional planar diffusion ?ame quenching.Top:reaction zone extremities interacting with vortices [11].Bottom:Quenching of planar trailing diffusion ?ame.
790V.FAVIER AND L.VERVISCH
emerge later after quenching,the reaction rate levels are well above those of a strained laminar diffusion ?amelet with the same scalar dissipa-tion rate.However,this burning rate is of the order of the one found in a diffusion ?ame close to quenching and also in a stoichiometric planar premixed ?ame.
The analysis of the scalar dissipation rate at quenching suggests that one should distinguish between the amount of scalar dissipation rate necessary to quench the diffusion ?ame ?q ,and,?q Ed ,the value measured at a quenched location bordering a reaction zone (Fig.3).In our sim-ulations,where unsteadiness effects at quench-ing are intentionally weak,the value ?q that should be applied to transition from burning to quenching is of the order of ?*q that predicted by ?amelet theory.In particular when ???*q ,quenching is always observed.However,once a hole exists in the reaction zone,due to multi-dimensional ?uxes of heat and species,?q Ed ,the level of scalar dissipation rate necessary to maintain quenching and increase the ?ame hole,is always lower than ?*q .This result is in agreement with experimental observations in a counter-?ow ?ame with non-uniform strain rate mentioned above [29]and theoretical works
have discussed forward and backward propaga-tion of triple ?ames in ?ame holes [33].
In the subsequent sections,the numerics and the simulations are ?rst presented.Then,diffu-sion ?ame quenching is studied by using mean quantities obtained by integration over the com-putational domain,with special emphasis on the response of the burning rate and temperature to scalar dissipation rate variations.The ?ame hole creation and the cross-scalar dissipation rate ?,used to distinguish between diffusion and pre-mixed reaction zone extremities,are also dis-cussed.Finally in the light of the numerical results,the behavior of the ?ame and of the scalar dissipation rate at quenching are analyzed.PROBLEM FORMULATION AND NUMERICAL PROCEDURE
A variety of model problems may be used to study quenching of diffusion ?ames [17,34].Two dimensional diffusion ?ame quenching may,for instance,be observed in a planar ?ame/vortex interaction,where a pair of vorti-ces are sent towards a planar ?ame.The ?ame becomes curved and extinction develops at a point located along the centerline of the vortex pair.Unsteadiness and curvature effects can be addressed carefully in this con?guration [7].As in the counter-?ow ?ame with a non-uniform distribution of scalar dissipation rate [29],our objective is to restrict the study to the development of quenching of a planar diffusion ?ame.To study ?amelet quenching without curvature of the stoichiometric line,we have retained a Direct Numerical Simulation (DNS)database initially developed to focus on triple ?ame/vortex interaction [11].The results of these interactions have previously been ana-lyzed to help understanding of non-premixed turbulent ?ame stabilization,the outcome being a diagram delineating conditions for upstream and downstream movement of a triple ?ame submitted to unsteady micromixing.In [11]it was shown that there exists a set of control parameters of the simulations for which,after interaction of the triple ?ame with vorticity,the trailing diffusion ?ame is subjected to a high level of strain and scalar dissipation rate leading to quenching of a two-dimensional planar
diffu-
Fig.3.Schematic of two scalar dissipation rates “at quench-ing”.Transition from burning to quenching occurs for ???*q .At the extremity of reaction zone bordering quenched location ???q Ed ??*q .
791
DIFFUSION FLAME QUENCHING
sion?amelet(Fig.2,bottom).The focus is on this part of the simulation where quenching of the trailing diffusion?ame appears.
The simulations are performed by using DNS tools[10,35–37,38]developed after previously established methods[39].The fully compress-ible Navier Stokes equations are solved by using a sixth order PADE scheme[40],combined with third order Runge–Kutta time stepping and Navier Stokes characteristic boundary condi-tions(NSCBC)[41].
The diffusion?ame is initially stabilized within a two-dimensional domain following a procedure proposed in[36].The computations are with a Zel’dovich number??8and a heat release parameter??(T burnt?T fresh)/T burnt?0.8,where T fresh and T burnt denote the temper-atures on the two sides of a reference stoichio-metric unstrained premixed front used to gen-erate the triple?ame.The reaction rate of the single-step global chemistry is given by an Ar-rhenius law.The Lewis and Schmidt numbers are set to unity,and for simplicity the initial triple?ame is symmetrical,Z s?0.5.The streamwise component of the velocity u is equal to the triple?ame propagation velocity S TF o,and the pair of vortices is convected towards the triple?ame to interact with https://www.sodocs.net/doc/159800021.html,ter and further downstream,quenching of the trailing diffusion ?ame occurs.
Early in the simulation,the partially pre-mixed front is convected upstream of its initial location.Then the planar diffusion?ame is squeezed by the vortices(Fig.4a–b),quenching occurs and two edge?ames bordering the?ame hole are formed(Fig.4c–e).Later,at the tip of the edge?ame located on the right,a growth in the reaction zone thickness is observed(Fig.4f). How this modi?cation of the edge?ame topol-ogy is related to premixed combustion at the ?ame tip(Fig.4f)will be addressed later. After diffusion?ame quenching,the overall ?ame may be organized in three main parts (Fig.5).The response in mixture fraction space of the partially premixed front remaining from the initial triple?ame evolves on the mixing lines(Y F M?Y F,o Z and Y O M?Y O,o(1?Z)), whereas combustion occurs in a near-stoichio-metric,partially premixed,regime(Fig.5b).Y i denotes species mass fractions and Y i,o values in the feeding streams.Over the two reaction zone extremities resulting from quenching of the trailing diffusion?ame(Fig.5a),one is attached to the main body of the initial triple?ame(on the left),the other(on the right)features an edge?ame[42],followed by the diffusion?ame. For these points,the?ame structure in mixture fraction space reveals combustion in a diffusive regime(Fig.5c).In what follows,the scope of the study is restricted to a zone?of the computational domain,excluding the partially premixed front of the initial triple?ame(Fig.
4).For the simulated case,during diffusion ?ame straining and at quenching,the vortices do not carry any hot gases and products remain-ing from their interaction with the partially premixed front(see Fig.5c).Thus,the free stream conditions of the diffusion?ame are pure fuel and air.
To compare the results of the full Navier Stokes equations with diffusion?ame theory,a reference laminar?amelet is calculated.In the simpli?ed case of a one-dimensional steady ?amelet,the temperature T?T(Z)is a func-tion of Z,and keeping only the higher order diffusive term,the governing equation of the ?amelet may be written[16,22,34]:
?
?2T
?Z2?
?˙T?0(1)
where?˙T is the burning rate.This equation is solved for the same chemistry and transport properties as in the full simulations,using for the mixture fraction space derivative the sixth order?nite difference scheme of the DNS[40]. The result is a laminar?amelet library chosen as a reference solution to analyze quenching and edge?ames(Fig.6).In?amelets featuring multiple species and reaction zones calculated with detailed chemistry,the quenching point may depend on the distribution?(Z)[34]. When solving Eq.1,single step chemistry is used and each?amelet is obtained for a unique value of?,measured under stoichiometric con-ditions.When?increases,the burning rate also increases with mass and heat transfer through the stoichiometric surface,until quenching oc-curs.The temperature continuously decreases with Da???1and the quenching point?*q is readily obtained from the?amelet calculations (Fig.6).
792V.FAVIER AND L.VERVISCH
The reference quenching scalar dissipation rate ?*q ,its corresponding quenching length
?*q ??D /?*q ,the burning rate ?
˙T Diff and the scalar dissipation rate ?Diff both measured in the unperturbed trailing diffusion ?ame are chosen
as control parameters of the DNS.?*q ,?Diff ?1
,and ?˙T Diff are used to normalize length,time and burning rate,respectively.In the results to be presented,t ?0corresponds to the instant at which ?starts to increase in the trailing diffu-sion ?ame (Fig.4a).The vortices are character-ized by their radius R ,velocity v ?and the length l v (Fig.2).The reported simulation has been
performed by using a representative condition where diffusion ?ame quenching is found:
?*q
?Diff ?100,l v
?*q ?12,R
*q
?5,v ?
*q *q
?40(2)
Varying these numbers changes the time and
the streamwise position at which quenching occurs,but does not fundamentally modify the presented results.As shown thereafter,this set of parameters limits unsteadiness effects via
a
Fig.4.Isolines of burning rate and vorticity (dash line)for six successive times.(a,b):squeezed diffusion ?ame.(c,d,e,f):edge ?ames.Frame denotes the studied domain ?.
793
DIFFUSION FLAME QUENCHING
suf?ciently slow increase of ?,allowing the diffusion ?ame to adjust in the ?rst phase of quenching and to behave as a steady strained ?ame.The emergence of the ?rst extinction is then easily characterized.
MEAN FLAME PROPERTIES AT QUENCHING
The time evolution of averaged quantities,Q
?,obtained from integration over the domain ?of
streamwise length L are ?rst discussed.Q
?is computed as:
Q
??t ????
Q ?x ,y ,t ?dx d y
L
?
??
Q ??,y ,t ?t o ?d y
(3)
where the properties in the trailing diffusion ?ame (x 3?,Fig.2)are chosen to normalize Q
?(t ).When the ?ame starts to be pinched,the vorticity ?eld modi?es the mixture and in-creases the amount of heat released.The in-crease of the averaged scalar dissipation rate ?
?
Fig.5.(a):Isolines of burning rate (lines)and fuel mass fraction (dashed lines),time of Fig.4d.(b):Pro?les of fuel mass fraction versus mixture fraction for the upstream triple ?ame.Positions in (a)1:line,2:dashed line,3:dotted line,4:triangles,5:circles.(c):For the edge-?ame.Positions in (a)6:line,7:dashed line,8:triangles,9:circles,10:stars.
794V.FAVIER AND L.VERVISCH
(Fig.7a,dashed line)is accompanied by an
increase of the mean burning rate ??˙
T (Fig.7a,full line).However,before ??reaches its maxi-mum,??˙
T features a plateau and suddenly drops (t ?0.3),indicating local quenching of the diffusion ?ame (Fig.4b–c and Fig.7a).From this instant a hole exists in the ?ame,??slowly
decays and ??˙
T is almost constant (t ?0.4).Highly strained diffusion combustion induces a
decrease of the partially premixed fraction ?
??Y F Y O until quenching appears (Fig.7b,dashed curve t ?0.2).Then,the weakly varying mean burning rate (Fig.7a,dashed curve t ?0.3)follows a growth in Y F Y O ,indicating some partial premixing of the reactants at the
quenched point.During the whole process ??˙
T ?1(Fig.7a),showing that a planar diffusion ?ame featuring a hole may release,on average,more heat than a ?ame at low strain.Because a high burning rate coexists in the simulation with
high straining (inducing strong heat transfer)T
??1(Fig.7b,line).
FLAME HOLE GENERATION
Instantaneous and local quantities are now an-alyzed.At the tip of the initial and unperturbed triple ?ame,a stoichiometric kernel exists and ensures the stabilization of the entire structure.Figure 8a (full line)shows the burning rate ?˙T
on the stoichiometric line of the triple ?ame.
The triple point is determined by the maximum value of ?˙T (x ?27),whereas a lower burning rate is found in the trailing diffusion ?ame (x ?35).A reaction rate pro?le is also reported for the same mixture,but through a stoichiometric planar pre-mixed ?ame (Fig.8a,dashed line).As expected for these parent ?ames [10,36,37,43–46],the peak values ?*T of the burning rate are of the same order and close to those of ?˙T observed at quenching of the ?amelet (Fig.6,circle).
When the partially premixed front interacts with the vortices it is convected upstream and the reaction zone burning in a diffusive regime is subjected to a high level of scalar dissipation rate.The burning rate increases in the diffusion ?ame to reach a value of the order of the premixed reference value,?˙*T ,(Fig.8b,full line,x ?24).Subsequently,chemistry cannot keep up with the tremendous heat and mass ?uxes and ?˙T progressively drops at the location of the future quenching point (Fig.8b,dotted and dashed lines),leading to a non-monotonic dis-tribution of ?˙T .The extinction point,where the reaction rate progressively decays,is always surrounded by two zones maintaining a burning rate of the order of the reference premixed value ?˙*T (Fig.8b,circle).Thus,during the two-dimensional quenching process,a ?ame hole and two points of high burning rate
simul-
Fig. 6.Maximum normalized temperature (triangles)and burning rate (circles)versus in-verse of normalized scalar dissi-pation rate ?.
795
DIFFUSION FLAME QUENCHING
taneously appear,with ?˙T ??˙*T .The ?nal outcome is a hole in the diffusion ?ame (Fig.8b,circle,20?x ?30)the length of which increases with time,the edge ?ames moving away from the high strained locations.The quenched zone is,therefore,limited by two intensely burning edge ?ames.During all the process leading to ?ame extinction,the amount of heat released is well above the characteristic level measured in the initial diffusion ?ame,?˙T being similar to the value ?˙*T observed in a
premixed ?ame or in a diffusion ?ame close to
quenching (Fig.6,circle).
THE CROSS SCALAR DISSIPATION RATE ?
Under stoichiometric conditions,at the zones bordering the quenched locations,the increase in Y F Y O leads to favorable conditions for par-tially premixed combustion.In the
quenching
Fig.7.(a):line,time evolution of
averaged burning rate ??˙T
.Dashed line,scalar dissipation rate ??.(b):
line,temperature T
?.Dashed line,product of mass fractions ?
??Y F Y O .
796V.FAVIER AND L.VERVISCH
process,the product of reactant mass fractions ??Y F Y O is useful to understand the transition from diffusion ?ame to partially premixed com-bustion.We ?rst analyze ?in the initial unper-turbed triple ?ame,where the ?distribution is decomposed in three distinct zones.In the feeding streams ??0.Before combustion at the partially premixed front,mixing occurs in a chemically frozen ?ow and transverse diffusion starting at the end of the splitter plate generates
non-zero values of ?.In this zone,??Y F M Y O M
?Y F ,o Y O ,o Z (1?Z ).
Across the partially premixed front,the con-sumption of reactants decreases ?according to the local equivalence ratio of the partially pre-mixed ?ame.Through the partially premixed ?ame,the quantity
c ?1?
?
Y F ,o Y O ,o Z ?1?Z ?
(4)
Fig.8.Normalized burning rate versus normalized axial x posi-tion.(a):line,initial triple ?ame.Dashed line,planar premixed ?ame.(b):line,squeezed diffu-sion ?ame (see Fig.4a).Dotted and dashed lines,?ame hole cre-ation for two successive times (see Fig.4b).Circles,edge ?ame (see Fig.4c).
797
DIFFUSION FLAME QUENCHING
de?nes a progress variable along the iso-Z surface,other de?nitions of c are possible for such partially premixed ?ames [47].Under stoi-chiometric conditions ??Y F ,o Y O ,o Z s (1?Z s )(c ?0in fresh gases)upstream of the ?ame.For the weak curvature of the partially pre-mixed front,because of the existence of a fully burning premixed kernel at the triple point,?falls to ??0(c ?1in burnt gases)(Fig.9).Finally in the trailing diffusion ?ame,because of ?nite rate chemistry ?may increase again,whereas a diffusion ?ame featuring in?nitely fast chemistry would be characterized by ??0.Nonetheless,eventual quenching of the trailing diffusion ?ame,followed by chemically frozen mixing,would lead to ?values of the order of those observed in the mixing zone upstream of the triple ?ame.From the balance equations for Y F and Y O ,an equation for ?is obtained:??
?t ?u ????1?
????D ????2D ?Y F ??Y O ??
???˙F Y O ??˙O Y F ?(5)
where ?˙i is the consumption rate of species i (?˙i ?0),and the usual notation is otherwise adopted.When no reaction occurs in the diffu-sive system,in Eq.5the term ???2D ?Y F ??Y O reduces to 2?and the ?bal-ance equation reads:
???t ?u ????1
?
????D ????2?(6)
As expected,the scalar dissipation rate ??
D ??Z ?2(micromixing)is the only source of ?in pure mixing problems.In Eq.5,?represents micromixing effects in both premixed and non-premixed systems.One may write ???2D ??Y F ???Y O ?n F ?n O ,where n i ???Y i /??Y i ?denotes the normal vector to the iso-Y i surface pointing towards small Y i values (Fig.10).In diffusion combustion,fuel and oxidizer are on both sides of the reaction zone with n F ?n O ?0[14],??0is a source since contact between the species increases mixing and hence also ?(??2?for strong ?nite rate chemistry).On the other hand,for a premixed system n F ?n O ?0and ??0acts as a sink because,for a premixed mixture,?promotes mixing contact between premixed fresh gases and burnt products,contributing to the decay of ??Y F Y O and to the increase of the progress vari-able c when crossing a reaction zone (Eq.4).Thus,?is found to play two different roles depending on the combustion regime and is therefore an interesting candidate to distinguish between diffusion and partially premixed com-bustion.?may be called “the cross-scalar dis-sipation rate”.In a non-premixed system,neg-ative values of ?are a direct indicator of the existence of partially premixed combustion and of the appearance of triple ?amelets.
Figure 11a presents the centerline distribu-tion of ?for various times,ranging from the initial steady triple ?ame up to the quenched diffusion ?ame.In the steady initial ?ame (Fig.11a,circle),??0,except in the vicinity of the partially premixed front where ??0,con?rm-ing that triple ?ame combustion is characterized by a local negative cross-scalar dissipation rate.During the development of quenching,because of intense mixing,??rst increases in the
?ame
Fig.9.Partially premixed fraction ?(line)and progress variable c (dashed line)versus normalized axial x position along the centerline of the initial triple
?ame.
Fig.10.Sketch of two types of edge ?ames.??0and ???:diffusion combustion.??0and ???/2:partially premixed combustion.
798V.FAVIER AND L.VERVISCH
hole,with ??0for all the studied domain ?(Fig.11a,dash).The edge ?ame observed at the diffusion ?ame extremity is then controlled by diffusion combustion (Fig.4d).Later in the simulation (Fig.4f),the diffusion ?ame extrem-ity features ??0(Fig.11a,dotted line and line),indicating the existence of partially pre-mixed combustion at the zone bordering the quenched location.
Introducing the angle ?between n F and n O ,the cross scalar dissipation rate may be written ???2D ??Y F ???Y O ?cos (?).Diffusion com-bustion would correspond to ???,whereas for ???/2premixed combustion may be expected (Fig.10).In the initial steady triple ?ame,the redirection of the species transport towards the partially premixed front leads to a strong de-crease of ?in the vicinity of the triple point (Fig.11b,circle).During quenching,?progressively decays leading to values below ?/2along with ?
?0and partially premixed combustion (Fig.11b,dash,dotted and line curves).
The change in sign of ?is also associated with a change of the edge ?ame topology.For edge ?ames with a reaction zone thickness of the order of the reaction zone observed in the diffusion ?ame (Fig.4d),??0and ???indicates combustion in a diffusive regime.However,when a premixed kernel emerges (Fig.4f),the thick-ness of the reaction zone extremity is greater than that of the trailing diffusion ?ame,then ??0and ???/2(Fig.10).Hence,the edge ?ame has evolved into a triple ?ame involving propagation mechanisms [36].SCALAR DISSIPATION RATE AT QUENCHING
We now focus on the time evolution of the main control parameters of the diffusion ?ame (Y F ,Y O ,T ,?˙T ,and ?),following an edge ?ame in the domain ?.The analysis employs lagrangian time evolution of these quantities at the posi-tion x q (t )of the quenching front located on the right,when it moves downstream away from large ?(Fig.4).The location of x q (t )is deter-mined from those where the maximum value of ?˙T is measured in the diffusion ?ame.In the ?rst phase of the interaction,x q (t )corresponds to the future quenching point (x q (t )?24in Fig.8b),then it progressively moves down-stream,with the maximum value of the reaction rate locating the edge ?ame,close to the ?ame hole (24?x q (t )?35in Fig.8b).
Figure 12(triangle)shows the lagrangian time evolution of the burning rate ?˙T (x q )(Fig.12a),the temperature T (x q )(Fig.12b),scalar dissipation rate ?(x q )(Fig.12c)and fuel mass fraction Y F (x q )(Fig.12d).Both T (x q )and Y F (x q )have weak variations.The burning rate varies with the scalar dissipation rate up to the decay of ?(x q ),which occurs at t ?0.6.This allows the reaction zone extremity to develop a partially premixed front.Then,independently of the level of ?,?˙T (x q )takes a value close to the reference burning rate ?˙*T ,obtained in the stoichiometric planar premixed ?ame,or in the initial triple ?ame stabilizing combustion (Fig.8a).
Along the stoichiometric line,two
different
Fig.11.(a):Normalized ?.(b):angle ?versus normalized axial x position.Circle:initial triple ?ame.Long-dash:edge ?ame at time of Fig.4d.Dotted line:at time of Fig.4f.Line:later in time.
799
DIFFUSION FLAME QUENCHING
?xed locations x o and x 1are further considered.The location x o ?x q (t o )?24is where the transition from burning to quenching is ?rst observed (Fig.8,dashed line)at t ?t o ?0.2(Fig.12a,full line).The development of the hole in the diffusion ?ame leads to a quenching front originated at x o and crossing,at t ?t 1?0.7(Fig.12a,circle),the further downstream location x 1?x q (t 1)?34(Fig.8,dotted line).Figure 12a–b shows that at both x o (line)and x 1(circle),the burning rate increases before a rapid decay down to quenching,also visible on T .After extinction,pure mixing develops with Y F ?Z s (Fig.12d,full line and circle).These two points undergo two different quenching mechanism,x o characterizes a local transition of the diffusion ?ame from burning to quench-ing,whereas at x 1backward propagation of a quenching front occurs along the stoichiometric line.At x 1,the burning rate slowly increases to change continuously from diffusion to premixed combustion (Fig.12a,circle).Just before the strong decay of the burning rate,the maximum values of ?˙T collected at both locations x 0and x 1are equal within 5%and of the order of ?˙*T (Fig.12a,full line at t ?0.2and circle at t ?0.7).In contrast,the accompanying scalar dis-sipation rates differ by 25%(Fig.12c,full line at t ?0.2and circle at t ?0.7),suggesting that primary extinction at x o ,and,extinction by backward edge ?ame propagation are two dif-ferent mechanisms that cannot be addressed by using a unique quenching scalar dissipation rate.To help discriminate between a local transi-tion from burning to quenching (x o )and back-ward propagation of a reaction zone (x 1)
(Fig.
Fig.12.(a):Time evolution of normalized burning rate.(b):Temperature.(c):Scalar dissipation rate.(d):Fuel mass fraction.Triangle:following reaction zone extremity x q (t ).Line:location of primary quenching x o ?24.Circle:further downstream x 1?34.
800V.FAVIER AND L.VERVISCH
3),the responses of the burning rate and of the temperature are collected along the stoichio-metric line and plotted versus the inverse of the scalar dissipation rate.Four successive times are considered on Fig.13,the results are also compared with the reference one-dimensional counter ?ow ?ame solution given by Eq.1(Fig.6).Before quenching and during the ?rst phase of the creation of the hole in the ?ame,a perfect agreement is observed between the ?amelet (Eq.1)and the real ?ame response ?˙T (?)(Fig.13a–c).The maximum burning rate increases with ?until the quenching point is reached,that is,for ???*q ,the ?amelet quenching scalar dissipation rate.Figure 12a (line)shows that at x o ,the time needed to change from burning to quenching is ?t ?0.05,whereas ?slowly evolves within ?t ?0.4,a time that is necessary for it to reach its maximum.This order of
magnitude difference between (1/?T )/(??T /?t )and (1/?)(??/?t ),along with the agreement between the ?amelet response and the full simulation,are indicators of weak effects of unsteadiness.
Once a reaction zone extremity exists,the peak value of the burning rate is always of the order of the premixed value ?˙*T ,while in the trailing burning zone,?˙T (?)agrees with the ?amelet behavior.However,the edge ?ame escaping from high strain cannot sustain values of ?greater than ?q Ed ??*q ???(x q (t )),leading to a scalar dissipation rate at the reac-tion zone extremity ?q Ed smaller than ?*q (Fig.13b–d).Therefore,even when the primary ex-tinction is fully characterized by ?amelet theory,the succeeding ampli?cation of quenching through the growth of ?ame holes is controlled by a distribution of scalar dissipation rates ?q
Ed
Fig.13.Normalized centerline burning rate (a,b)and temperature (c,d)versus inverse of normalized scalar dissipation rate.Circle:?amelet theory.(a,c):dashed line,time of Fig.4b.Dotted line,time of Fig.4c.(b,d):line,time of Fig.4d.Line with triangle,time of Fig.4e.Line with square,time of Fig.4f.
801
DIFFUSION FLAME QUENCHING
linked to local partially premixed combustion,rather than by the single value ?*q .
Except when unsteadiness was explicitly quanti?ed by using oscillating one-dimensional ?amelets [4,48],in direct numerical simulation (DNS)or in experiments of non-premixed tur-bulent ?ames,the exact distinction between ?*q and ?q Ed ,the two scalar dissipation rates “at quenching”,is a priori non-trivial (Fig.3).
This last result possesses implications for turbulent combustion modeling.In many mod-els the area of burning stoichiometric surface is determined from the condition ???*q .Be-cause after primary quenching,partially pre-mixed combustion leads to ?ame holes that may spread for ???q Eq ??*q ,other ingredients may be needed to complement the burning criterion ???*q .CONCLUSION
Numerical simulations of a two-dimensional pla-nar diffusion ?ame (single step chemistry and unity Lewis number)undergoing enhanced strain and mixing suggest that quenching mech-anisms may be decomposed in two stages.For weak effects of unsteadiness,extinction ?rst ap-pears for a scalar dissipation rate ?*q that is per-fectly predicted by one-dimensional steady ?ame-let theory.Once a hole exists in the ?ame,edge ?ames appear at the extremity of the reaction zones.At these new ?ame tips,diffusion com-bustion progressively evolves into partially pre-mixed combustion.The balance equation for the product of species mass fraction Y F Y O involves a cross scalar dissipation rate ???2D ?Y F ??Y O the sign of which is a direct indicator of the two combustion regimes (??0:diffusion,??0:premixed).After primary extinction,the length of the quenched stoichiometric line grows,even for scalar dissipation rate levels lower than ?*q .This ?nding is associated with backward propa-gation of the edge ?ames and may be of interest in turbulent combustion,when modeling ?nite rate chemistry and quenching of the iso-stoichi-ometric surfaces.In other words,once ?ame quenching has started to develop,the scalar dissipation rate controlling the growth of the ?ame hole differs from that which should be applied to ?rst quench the ?ame.The quench-
ing scalar dissipation rate measured at the re-action zone extremity is always smaller than the reference ?amelet quenching value.The peak burning rate of a stoichiometric and planar premixed ?ame emerges as a reference value recovered in the diffusion ?ame close to quenching and at the edge ?ame.The exact impact of complex chemistry and transport (non-unity Lewis number and differential mass diffusion)on this last result is an open question.
REFERENCES
1.Williams,F.A.,Annu.Rev.Fluid Mech .,3:171–188(1971).
2.Lin ?a ′n,A.,Acta Astronautica ,1007(1974).
3.Chang,C.H.H.,Dahm,W.J.A.,and Trygvason,G.,Phys.Fluid ,3:1300–1311(1991).
4.Darabiha,N.,Combust.Sci.Technol .,86:163–181(1992).
5.Mell,W.E.,Nilsen,V.N.,Kosa ′ly,G.,and Riley,J.J.,Combust.Sci.and Technology ,91:179–186(1993).
6.Delhaye,B.,Veynante,D.,and Candel,S.,Theoretical and Computational Fluid Dynamics ,6:67–87(1994).
7.
Cuenot,B.,and Poinsot,T.J.,Twenty-Fifth Symp.(Int.)on Combustion ,The Combustion Institute,Pittsburgh,1994,pp.1383–1390.
8.Mahalingam,S.,Chen,J.H.,and Vervisch,L.,Com-bust.Flame ,102:285–297(1995).
9.Lee,Y.Y.,and Pope,S.B.,Combust.Flame ,101:501–528(1995).
10.
Domingo,P.,and Vervisch,L.,Twenty-Sixth Symp.(Int.)on Combustion ,The Combustion Institute,Pitts-burgh,1996,pp.233–240.
11.
Favier,V.,and Vervisch,L.,Twenty-Seventh Sympo-sium (Int.)on Combustion ,The Combustion Institute,1998,pp.1239–1245.12.Be ′dat,B.,Egolgopoulos,F.,and Poinsot,T.,Combust.Flame ,119:69–83(1999).
13.
Nayagam,V.,Balasubramaniam,R.,and Ronney,P.D.,Combust.Theory and Modelling ,3(4):727–742(1999).
14.
Yamashita,H.,Shimada,M.,and Takeno,T.,Twenty-Sixth Symposium (Int.)on Combustion ,The Combus-tion Institute,1996,pp.27–34.
15.Masri, A.R.,Bilger,R.W.,and Dibble,R.W.,Combust.Flame ,73:261–258(1988).
16.
Bray,K.N.C.,and Peters,N.,in Turbulent Reacting Flows (P.A.Libby and F.A.Williams,Eds.),Aca-demic Press London,1994,pp.63–113.
17.Vervisch,L.,and Poinsot,T.,Annu.Rev.Fluid Mech .,30:655–692(1998).
18.
Vervisch,L.,Twenty-Eighth Int.Symposium on Com-bustion ,The Combustion Institute,Pittsburgh,2000,pp.11–24.
19.
Sung,C.J.,Liu,J.B.,and Law,C.K.,Combust.Flame ,102:481–492(1995).
802
V.FAVIER AND L.VERVISCH
20.Sun,C.J.,Sung,C.J.,Wang,H.,and Law,C.K.,
Combust.Flame,107(4):321–335(1996).
21.Bilger,R.W.,Ann.Rev.Fluid Mech.,21:101–135
(1989).
22.Williams,F.A.,Combustion Theory.The Benjamin/
Cummings Publishing Company,Inc,1985.
23.Pope,S.B.,Prog.Energy Combust.Sci.,11:119–195
(1985).
24.Kollmann,W.,Theor.and Comp.Fluid Dynamics,
1:349–285(1990).
25.Dopazo,C.,in Turbulent Reacting Flows(P.A.Libby
and F.A.Williams,Eds.),Academic Press London, 1994,p.375–474.
26.Vervisch,L.,and Veynante,D.,Twenty-eighth Int.
Symposium on Combustion,2000,The Combustion Institute,Pittsburgh.
27.Peters,N.,Prog.Energy Combustion Sci.,10:319–339
(1984).
28.Swaminathan,N.,and Bilger,R.W.,Combust.Flame,
116(4):519–545(1999).
29.Shay,M.L.,and Ronney,P.D.,Combust.Flame,
112(1/2):112–171(1998).
30.Mun?iz,L.,and Mungal,M.G.,Combust.Flame,
111(1/2):16–31(1997).
31.Watson,K.A.,Lyons,K.M.,Donbar,J.M.,and
Carter, C. D.,Combust.Flame,119(1/2):199–202 (1999).
32.Buckmaster,J.,Combust.Sci.Tech.,115:41–68(1996).
33.Dold,J.W.,In Proceedings of the Zel’Dovich Memorial
(A.G.Mershanov and S.M.Frolov,Eds.),Institute of
Structural Macrokinetics,Chernogolovka,Russia, 1994,pp.7–19.34.Peters,N.,Turbulent Combustion.Cambridge Univer-
sity Press,2000.
35.Guichard,L.,PhD thesis,Universite′de Rouen,
France,1999.
36.Ruetsch,G.R.,Vervisch,L.,and Lin?a′n,A.,Phys.
Fluids,6:1447–1454(1995).
37.Ghosal,S.,and Vervisch,L.,J.Fluid Mech.,415:227–
260(2000).
38.Re′veillon,J.,and Vervisch,L.,Combust.Flame,121:
75–90(2000).
39.Poinsot,T.,Candel,S.,and Trouve′,A.,Prog.Energy
Combust.Sci.,12:531–576(1996).
40.Lele,S.K.,https://www.sodocs.net/doc/159800021.html,put.Phys.,103:16–42(1992).
41.Poinsot,T.,and Lele,S.K.,https://www.sodocs.net/doc/159800021.html,put.Phys.,1:104–
129(1992).
42.Buckmaster,J.,and Weber,R.,Proceedings of the26th
Symp.(Int.)on Combustion.The Combustion Institute, Pittsburgh,1996.
43.Dold,J.W.,Combust.Flame,76:71–88(1989).
44.Kioni,P.N.,Rogg,B.,Bray,K.N.C.,and Lin?a′n,A.,
Combust.Flame,95:276(1993).
45.Echekki,T.,and Chen,J.H.,Combust.Flame,114:
231–245(1998).
46.Plessing,T.,Terhoeven,P.,Peters,N.,and Mansour,
M.S.,Combust.Flame,115:335–353(1998).
47.Poinsot,T.J.,Veynante,D.,Trouve′,A.,and Ruetsch,
G.,In Studying turbulence using numerical databases—
VI,pages111–136.CTR,Stanford U.,1996.
48.Im,H.G.,Chen,J.H.,and Chen,J.-Y.,Combust.
Flame,118:204–212(1999).
Received28April2000;revised6October2000;accepted10 October2000
803
DIFFUSION FLAME QUENCHING
